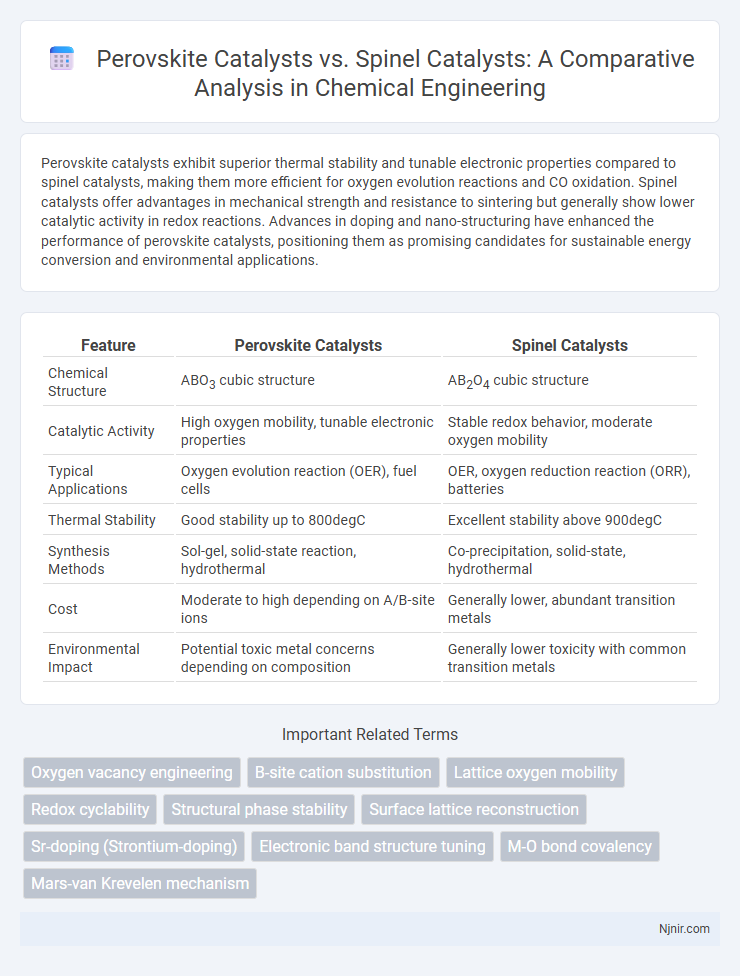
Perovskite catalysts exhibit superior thermal stability and tunable electronic properties compared to spinel catalysts, making them more efficient for oxygen evolution reactions and CO oxidation. Spinel catalysts offer advantages in mechanical strength and resistance to sintering but generally show lower catalytic activity in redox reactions. Advances in doping and nano-structuring have enhanced the performance of perovskite catalysts, positioning them as promising candidates for sustainable energy conversion and environmental applications.
Table of Comparison
| Feature | Perovskite Catalysts | Spinel Catalysts |
|---|---|---|
| Chemical Structure | ABO3 cubic structure | AB2O4 cubic structure |
| Catalytic Activity | High oxygen mobility, tunable electronic properties | Stable redox behavior, moderate oxygen mobility |
| Typical Applications | Oxygen evolution reaction (OER), fuel cells | OER, oxygen reduction reaction (ORR), batteries |
| Thermal Stability | Good stability up to 800degC | Excellent stability above 900degC |
| Synthesis Methods | Sol-gel, solid-state reaction, hydrothermal | Co-precipitation, solid-state, hydrothermal |
| Cost | Moderate to high depending on A/B-site ions | Generally lower, abundant transition metals |
| Environmental Impact | Potential toxic metal concerns depending on composition | Generally lower toxicity with common transition metals |
Introduction to Perovskite and Spinel Catalysts
Perovskite catalysts, characterized by the general formula ABO3, exhibit a versatile crystal structure that enables exceptional catalytic activity in various oxidation and reduction reactions. Spinel catalysts possess a distinctive AB2O4 structure featuring tetrahedral and octahedral sites, promoting enhanced stability and oxygen ion mobility crucial for catalytic performance. Both catalyst types are integral to energy conversion and environmental applications due to their tunable electronic properties and abundant elemental compositions.
Structural Differences: Perovskite vs Spinel
Perovskite catalysts feature a distinctive ABO3 crystal structure where a larger A-site cation and smaller B-site cation form a cubic lattice, enabling versatile ionic substitutions that enhance catalytic activity. Spinel catalysts possess a AB2O4 structure with a cubic close-packed oxygen framework, where A-site cations occupy tetrahedral sites and B-site cations reside in octahedral sites, influencing electronic conductivity and surface properties. These structural differences impact oxygen mobility, redox behavior, and catalytic performance in oxidation and reduction reactions crucial for energy and environmental applications.
Synthesis Methods for Perovskite and Spinel Catalysts
Perovskite catalysts are commonly synthesized via sol-gel, solid-state reaction, and combustion methods, enabling precise control over stoichiometry and particle size. Spinel catalysts are typically prepared using co-precipitation, hydrothermal synthesis, and mechanochemical approaches, facilitating homogeneous metal ion distribution and enhanced surface area. Optimization of temperature, pH, and precursor concentration during synthesis significantly influences the catalytic activity and structural stability of both perovskite and spinel materials.
Surface Properties and Active Sites
Perovskite catalysts exhibit highly tunable surface properties due to their flexible ABO3 crystal structure, allowing precise control over oxygen vacancies and metal cation arrangements that enhance catalytic activity. Spinel catalysts, characterized by the AB2O4 structure, provide robust surface stability and a distinctive distribution of active sites primarily at tetrahedral and octahedral metal ion positions, which influence redox behavior and adsorption characteristics. The density and accessibility of active sites on perovskites often surpass those on spinels, leading to superior performance in reactions such as oxygen evolution and reduction due to enhanced electron mobility and surface reactivity.
Catalytic Performance in Industrial Reactions
Perovskite catalysts exhibit superior catalytic performance in industrial reactions such as methane reforming and oxygen evolution due to their flexible crystal structure and tunable electronic properties, enabling higher activity and stability. Spinel catalysts offer robust thermal stability and resistance to sintering, making them effective in high-temperature processes like selective oxidation and hydrodesulfurization. Comparative studies highlight that perovskites often achieve lower activation energies and enhanced catalytic turnover frequencies, whereas spinels provide longer operational lifetimes under harsh reaction conditions.
Thermal and Chemical Stability Comparison
Perovskite catalysts demonstrate superior thermal stability due to their robust crystal structure, maintaining catalytic activity at temperatures exceeding 800degC, compared to spinel catalysts which typically degrade above 700degC. Chemically, perovskites offer enhanced resistance to acidic and oxidative environments owing to their flexible ABO3 lattice, while spinels are more susceptible to phase transformations and surface oxidation under harsh chemical conditions. These differences make perovskites more suitable for high-temperature and chemically aggressive catalytic applications.
Oxygen Mobility and Redox Behavior
Perovskite catalysts exhibit superior oxygen mobility due to their flexible crystal lattice and the ability to accommodate oxygen vacancies, enhancing redox reactions critical for catalytic performance. Spinel catalysts, while stable and structurally robust, generally show lower oxygen diffusion rates because of their more rigid lattice framework, limiting oxygen ion transport during redox processes. The enhanced redox behavior of perovskites, driven by their variable oxidation states and oxygen vacancy formation, facilitates faster oxygen exchange compared to spinels, making them preferable for applications requiring high oxygen mobility.
Environmental Applications: Emission Control and Water Treatment
Perovskite catalysts exhibit high oxygen mobility and excellent redox properties, making them highly effective for emission control by catalyzing the oxidation of CO, NOx, and volatile organic compounds in automotive exhaust systems. Spinel catalysts, characterized by their robust thermal stability and specific cation distribution, are widely used in water treatment processes to degrade organic contaminants through advanced oxidation reactions. Both catalyst types enhance environmental remediation efforts by offering sustainable and efficient pathways for pollutant removal in air and water purification technologies.
Economic and Scalability Considerations
Perovskite catalysts are favored for their lower material costs and abundant raw materials, enhancing economic viability for large-scale applications compared to spinel catalysts, which often rely on more expensive or less abundant elements. Scalability of perovskite catalysts benefits from established synthetic routes that support mass production with consistent quality, whereas spinel catalysts may face challenges related to complex synthesis and limited availability of high-purity precursors. Economically, perovskites offer a better balance between performance and cost-efficiency, making them more suitable for industrial-scale catalysis.
Future Trends and Research Directions
Future trends in perovskite catalysts emphasize tunable electronic structures and enhanced oxygen vacancy formation for improved catalytic activity in energy storage and conversion applications. Research directions in spinel catalysts focus on doping strategies and nano-structuring to boost stability and selectivity in oxygen evolution and reduction reactions. Combining perovskite and spinel phases may lead to hybrid catalysts with synergistic properties for next-generation fuel cells and metal-air batteries.
Oxygen vacancy engineering
Oxygen vacancy engineering in Perovskite catalysts significantly enhances their catalytic activity and stability compared to Spinel catalysts by facilitating improved oxygen ion mobility and reactive sites.
B-site cation substitution
B-site cation substitution in Perovskite catalysts markedly enhances oxygen evolution reaction efficiency compared to Spinel catalysts by optimizing electronic structure and catalytic active sites.
Lattice oxygen mobility
Perovskite catalysts exhibit higher lattice oxygen mobility than Spinel catalysts, enhancing their efficiency in catalytic oxidation reactions and oxygen evolution processes.
Redox cyclability
Perovskite catalysts exhibit superior redox cyclability compared to spinel catalysts due to their flexible crystal structure enabling enhanced oxygen vacancy formation and reversible oxidation states.
Structural phase stability
Perovskite catalysts exhibit superior structural phase stability under high-temperature conditions compared to Spinel catalysts, enhancing their durability and catalytic performance in industrial applications.
Surface lattice reconstruction
Surface lattice reconstruction in perovskite catalysts enhances active site exposure and oxygen vacancy formation more effectively than in spinel catalysts, improving catalytic performance and stability.
Sr-doping (Strontium-doping)
Sr-doping enhances the oxygen vacancy concentration and catalytic activity of Perovskite catalysts more effectively than in Spinel catalysts, improving their performance in oxygen evolution and reduction reactions.
Electronic band structure tuning
Perovskite catalysts exhibit versatile electronic band structure tuning through A-site and B-site cation substitution, enabling enhanced catalytic activity compared to spinel catalysts, whose band structure modification is more limited due to their fixed AB2O4 configuration.
M-O bond covalency
Perovskite catalysts exhibit higher M-O bond covalency than spinel catalysts, enhancing their electronic conductivity and catalytic activity in oxygen evolution reactions.
Mars-van Krevelen mechanism
Perovskite catalysts exhibit superior Mars-van Krevelen mechanism performance compared to spinel catalysts due to their flexible oxygen lattice and higher oxygen vacancy mobility, enhancing redox cycles and catalytic efficiency.
Perovskite catalysts vs Spinel catalysts Infographic
 njnir.com
njnir.com