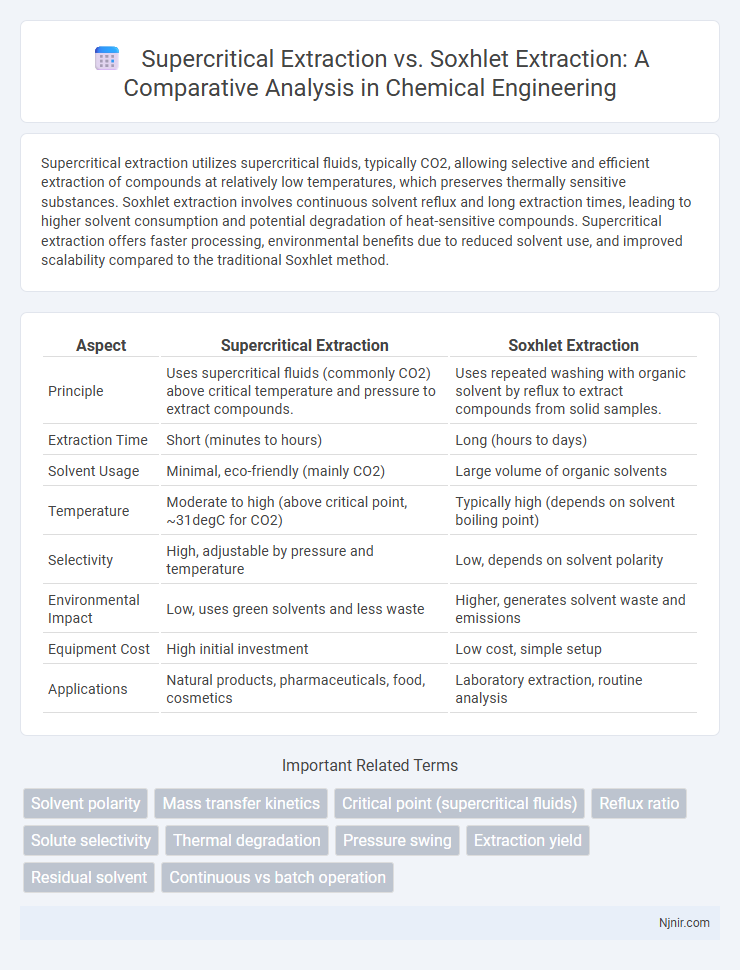
Supercritical extraction utilizes supercritical fluids, typically CO2, allowing selective and efficient extraction of compounds at relatively low temperatures, which preserves thermally sensitive substances. Soxhlet extraction involves continuous solvent reflux and long extraction times, leading to higher solvent consumption and potential degradation of heat-sensitive compounds. Supercritical extraction offers faster processing, environmental benefits due to reduced solvent use, and improved scalability compared to the traditional Soxhlet method.
Table of Comparison
| Aspect | Supercritical Extraction | Soxhlet Extraction |
|---|---|---|
| Principle | Uses supercritical fluids (commonly CO2) above critical temperature and pressure to extract compounds. | Uses repeated washing with organic solvent by reflux to extract compounds from solid samples. |
| Extraction Time | Short (minutes to hours) | Long (hours to days) |
| Solvent Usage | Minimal, eco-friendly (mainly CO2) | Large volume of organic solvents |
| Temperature | Moderate to high (above critical point, ~31degC for CO2) | Typically high (depends on solvent boiling point) |
| Selectivity | High, adjustable by pressure and temperature | Low, depends on solvent polarity |
| Environmental Impact | Low, uses green solvents and less waste | Higher, generates solvent waste and emissions |
| Equipment Cost | High initial investment | Low cost, simple setup |
| Applications | Natural products, pharmaceuticals, food, cosmetics | Laboratory extraction, routine analysis |
Introduction to Extraction Techniques in Chemical Engineering
Supercritical extraction utilizes supercritical fluids, typically CO2, as solvents to achieve efficient separation of compounds with enhanced selectivity and reduced solvent residues. Soxhlet extraction involves continuous solvent cycling through solid samples, allowing thorough extraction but often requiring longer times and larger solvent volumes. Both techniques are fundamental in chemical engineering for isolating target substances, with supercritical extraction offering environmentally friendly and scalable alternatives compared to traditional Soxhlet methods.
Overview of Supercritical Extraction
Supercritical extraction utilizes supercritical fluids, often carbon dioxide, to selectively dissolve and extract compounds with enhanced efficiency and purity compared to traditional methods like Soxhlet extraction. This technique operates at temperatures and pressures above the critical point of the solvent, enabling faster mass transfer and reduced solvent usage. Supercritical extraction is widely applied in pharmaceuticals, food processing, and natural product isolation due to its environmentally friendly nature and superior extract quality.
Fundamentals of Soxhlet Extraction
Soxhlet extraction relies on continuous solvent reflux and percolation to extract compounds from solid materials, allowing efficient recovery of bioactive substances through repeated washing. This technique uses solvents like hexane or ethanol heated to reflux, which dissolve target compounds and cycle them through the sample chamber repeatedly. The fundamental principle of Soxhlet extraction ensures thorough extraction by maintaining prolonged contact between fresh solvent and the sample, enabling effective separation without the need for continuous solvent replenishment.
Chemical Principles Behind Each Method
Supercritical extraction relies on the unique properties of supercritical fluids, typically carbon dioxide above its critical temperature and pressure, enabling enhanced solvent power and selective solute dissolution due to tunable density and diffusivity. Soxhlet extraction operates on continuous solvent reflux and percolation, where repeated washing with hot solvent dissolves target compounds based on differential solubility and thermal stability in conventional organic solvents. The chemical principle behind supercritical extraction is phase equilibrium manipulation in supercritical fluids, whereas Soxhlet extraction depends on solvent polarity and repeated solvent-solute interaction dynamics under boiling conditions.
Equipment and Process Design Comparison
Supercritical extraction utilizes high-pressure pumps, temperature-controlled vessels, and separators to maintain fluid conditions above critical points, enabling efficient solvent diffusion and selective extraction with minimal thermal degradation. Soxhlet extraction employs a simpler apparatus consisting of a heating mantle, extraction chamber, and condenser, relying on continuous solvent reflux for extended periods to dissolve target compounds. The supercritical method's precise control over pressure and temperature offers enhanced extraction efficiency and scalability, while the Soxhlet process features lower upfront equipment costs but longer extraction times and solvent consumption.
Solvent Selection and Environmental Impact
Supercritical extraction utilizes supercritical fluids like CO2, which are non-toxic, non-flammable, and leave no solvent residues, making it a more environmentally friendly option compared to Soxhlet extraction that relies on large volumes of organic solvents such as hexane or ethanol, which pose greater environmental and health risks. The solvent selection in supercritical extraction allows precise tuning of solvent density and selectivity, enhancing efficiency and minimizing waste, while Soxhlet extraction often requires longer extraction times and generates significant solvent waste. The sustainable appeal of supercritical extraction aligns with modern green chemistry principles, reducing hazardous emissions and solvent disposal challenges inherent in Soxhlet methods.
Efficiency and Yield of Target Compounds
Supercritical extraction offers higher efficiency and yield of target compounds compared to Soxhlet extraction due to its ability to penetrate materials more effectively using supercritical fluids like CO2, which enhances solubility and selectivity. Soxhlet extraction often requires longer extraction times and larger solvent volumes, resulting in lower efficiency and potential degradation of heat-sensitive compounds. Supercritical extraction also allows precise control over temperature and pressure, optimizing compound recovery and minimizing solvent residues.
Scalability and Industrial Applications
Supercritical extraction offers superior scalability for industrial applications due to its rapid processing times and ability to handle large volumes with precise solvent control, making it ideal for pharmaceuticals and food industries. Soxhlet extraction, while reliable for laboratory-scale extractions, faces limitations in scalability because of longer extraction cycles, high solvent consumption, and safety concerns in larger operations. Industrial applications favor supercritical extraction for environmentally friendly solvent use, reduced energy consumption, and enhanced extraction efficiency, supporting sustainable manufacturing practices.
Operational Costs and Energy Consumption
Supercritical extraction typically involves higher initial equipment costs but offers lower operational costs due to faster extraction times and reduced solvent usage, making it more energy-efficient than Soxhlet extraction. Soxhlet extraction consumes significantly more energy and solvents, as it relies on prolonged heating and continuous solvent reflux cycles. Over time, supercritical fluid extraction proves more cost-effective by minimizing solvent recovery and disposal expenses, alongside reduced energy consumption.
Future Trends and Innovations in Extraction Technologies
Supercritical extraction is gaining traction due to its eco-friendly solvents and ability to preserve bioactive compounds, making it a key focus in green chemistry innovations. Emerging trends highlight the integration of automation and real-time monitoring to enhance extraction efficiency and reproducibility. Advances in hybrid systems combining supercritical and Soxhlet techniques aim to optimize yield and selectivity for pharmaceutical and nutraceutical industries.
Solvent polarity
Supercritical extraction utilizes adjustable solvent polarity through pressure and temperature control, enabling selective extraction, whereas Soxhlet extraction relies on fixed solvent polarity determined by the choice of solvent.
Mass transfer kinetics
Supercritical extraction exhibits faster mass transfer kinetics than Soxhlet extraction due to enhanced solvent diffusivity and reduced viscosity under supercritical conditions, leading to more efficient solute solubilization and shorter extraction times.
Critical point (supercritical fluids)
Supercritical extraction operates above the critical point where fluids exhibit unique solvent properties, enabling efficient separation, whereas Soxhlet extraction relies on continuous solvent reflux without reaching supercritical conditions.
Reflux ratio
Supercritical extraction maintains a controllable and lower reflux ratio compared to Soxhlet extraction, resulting in higher efficiency and reduced solvent consumption.
Solute selectivity
Supercritical extraction offers superior solute selectivity compared to Soxhlet extraction by precisely tuning temperature and pressure to target specific compounds.
Thermal degradation
Supercritical extraction minimizes thermal degradation by operating at lower temperatures and controlled pressures, whereas Soxhlet extraction often causes higher thermal degradation due to prolonged exposure to elevated temperatures.
Pressure swing
Supercritical extraction utilizes pressure swing techniques to precisely manipulate solvent density and solubility, enhancing extraction efficiency compared to the constant pressure operation of Soxhlet extraction.
Extraction yield
Supercritical extraction typically achieves higher extraction yields than Soxhlet extraction due to its enhanced solvent diffusivity and tunable solvent properties under supercritical conditions.
Residual solvent
Supercritical extraction leaves significantly lower residual solvent levels compared to Soxhlet extraction, enhancing the purity and safety of the final product.
Continuous vs batch operation
Supercritical extraction operates as a continuous process enhancing efficiency and scalability, whereas Soxhlet extraction functions as a batch operation with limited throughput and longer processing times.
Supercritical extraction vs Soxhlet extraction Infographic
 njnir.com
njnir.com