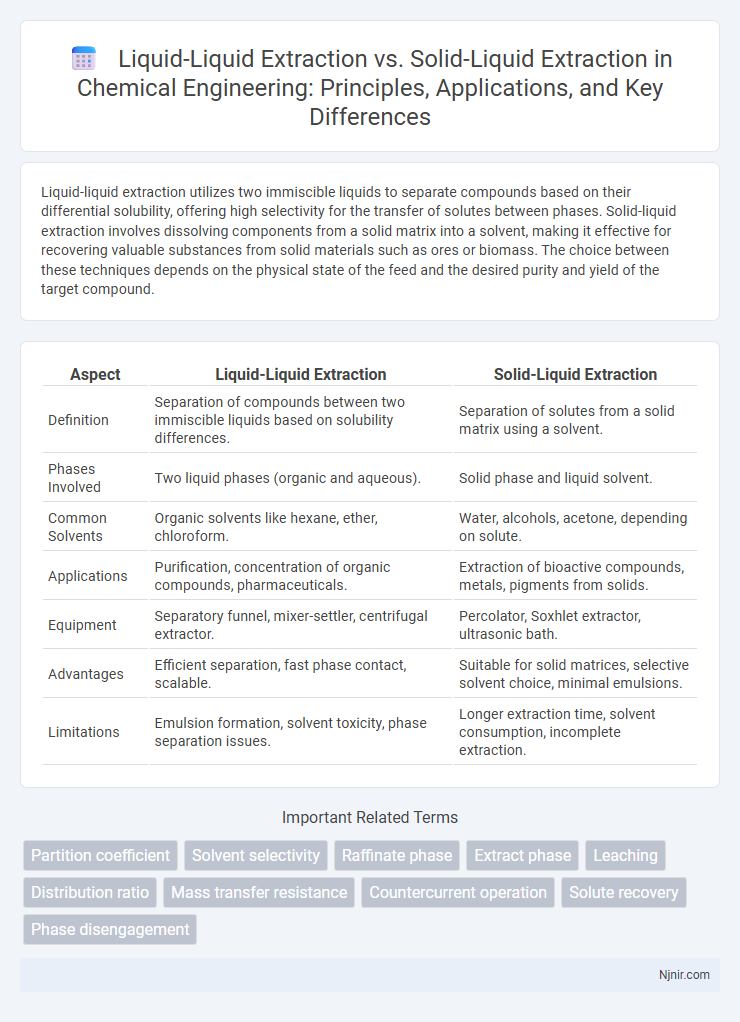
Liquid-liquid extraction utilizes two immiscible liquids to separate compounds based on their differential solubility, offering high selectivity for the transfer of solutes between phases. Solid-liquid extraction involves dissolving components from a solid matrix into a solvent, making it effective for recovering valuable substances from solid materials such as ores or biomass. The choice between these techniques depends on the physical state of the feed and the desired purity and yield of the target compound.
Table of Comparison
| Aspect | Liquid-Liquid Extraction | Solid-Liquid Extraction |
|---|---|---|
| Definition | Separation of compounds between two immiscible liquids based on solubility differences. | Separation of solutes from a solid matrix using a solvent. |
| Phases Involved | Two liquid phases (organic and aqueous). | Solid phase and liquid solvent. |
| Common Solvents | Organic solvents like hexane, ether, chloroform. | Water, alcohols, acetone, depending on solute. |
| Applications | Purification, concentration of organic compounds, pharmaceuticals. | Extraction of bioactive compounds, metals, pigments from solids. |
| Equipment | Separatory funnel, mixer-settler, centrifugal extractor. | Percolator, Soxhlet extractor, ultrasonic bath. |
| Advantages | Efficient separation, fast phase contact, scalable. | Suitable for solid matrices, selective solvent choice, minimal emulsions. |
| Limitations | Emulsion formation, solvent toxicity, phase separation issues. | Longer extraction time, solvent consumption, incomplete extraction. |
Introduction to Extraction Techniques in Chemical Engineering
Liquid-liquid extraction involves separating compounds based on their differing solubilities in two immiscible liquid phases, often used to isolate organic compounds from aqueous solutions. Solid-liquid extraction, also known as leaching, extracts solutes from solid matrices into a liquid solvent, commonly applied in natural product isolation and metal recovery. Both techniques optimize mass transfer principles in chemical engineering for efficient separation and purification processes.
Principles of Liquid-Liquid Extraction
Liquid-liquid extraction relies on the differential solubility of compounds between two immiscible liquid phases, commonly an aqueous and an organic solvent, to separate components based on their partition coefficients. The process involves equilibrating the mixture with the extracting solvent, allowing target solutes to preferentially distribute into the chosen phase, optimizing recovery and purity. Mass transfer efficiency and solvent selection, guided by solubility parameters and partition ratios, are critical to maximizing extraction yield and selectivity.
Fundamentals of Solid-Liquid Extraction
Solid-liquid extraction involves the transfer of solutes from a solid matrix into a liquid solvent based on differences in solubility and diffusion rates, enabling efficient separation of desired compounds. Key factors influencing this process include solvent polarity, particle size of the solid, temperature, and agitation, which enhance mass transfer and extraction yield. Unlike liquid-liquid extraction, which relies on immiscible liquid phases, solid-liquid extraction requires overcoming solid matrix barriers to optimize solvent penetration and solute dissolution.
Key Differences Between Liquid-Liquid and Solid-Liquid Extraction
Liquid-liquid extraction involves the transfer of a solute from one liquid phase to another immiscible liquid phase, commonly used for separating compounds based on solubility differences. Solid-liquid extraction extracts solutes from a solid matrix into a liquid solvent, typically applied in processes like caffeine extraction from coffee beans or herbal extractions. Key differences include phase states involved, solute transfer mechanisms, and typical application domains, with liquid-liquid extraction favoring immiscible liquid pairs and solid-liquid extraction relying on solvent penetration and solvation of solid-bound compounds.
Solvent Selection Criteria for Both Extraction Methods
Solvent selection criteria for liquid-liquid extraction emphasize immiscibility with the other liquid phase, high distribution coefficient for the target solute, low toxicity, and ease of solvent recovery. In solid-liquid extraction, solvents must exhibit strong solubility for the target compound, high penetration ability into the solid matrix, minimal swelling or degradation effects, and compatibility with subsequent processing steps. Both methods prioritize solvent selectivity, vapor pressure, environmental impact, and cost-effectiveness to optimize extraction efficiency and product purity.
Process Design Considerations
Liquid-liquid extraction requires precise control of phase ratio, interfacial area, and solvent selection to maximize mass transfer efficiency while preventing emulsion formation, making equipment design and solvent recovery critical. Solid-liquid extraction depends on particle size, solvent permeability, and agitation intensity, with careful optimization of contact time and temperature to enhance solute solubility and extraction yield. Process design must address scalability, ease of separation, and environmental impact for both techniques to ensure operational efficiency and sustainability.
Advantages and Limitations of Liquid-Liquid Extraction
Liquid-liquid extraction offers high selectivity and efficiency in separating compounds based on their solubility differences between two immiscible liquids, making it ideal for purifying pharmaceuticals and natural products. This method enables easy phase separation and rapid equilibrium but is limited by the potential formation of emulsions and the need for large volumes of often hazardous organic solvents. Despite its scalability and adaptability, liquid-liquid extraction can be less environmentally friendly and may require complex solvent recovery processes, impacting overall operational costs.
Advantages and Limitations of Solid-Liquid Extraction
Solid-liquid extraction offers advantages such as the ability to process solid materials without the need for dissolution, preserving the integrity of heat-sensitive compounds and enabling selective extraction based on solvent choice. This method is straightforward, cost-effective, and suitable for extracting bioactive compounds from plant matrices, but it is limited by slower mass transfer rates, potential solvent residue in the final product, and reduced efficiency with poorly soluble constituents. Unlike liquid-liquid extraction, solid-liquid extraction often requires longer processing times and may demand extensive solvent usage to achieve desired extraction yields.
Industrial Applications and Case Studies
Liquid-liquid extraction (LLE) is widely employed in the pharmaceutical and petrochemical industries for separating complex mixtures, such as recovering bioactive compounds or purifying crude oil fractions, due to its efficiency in handling immiscible liquid phases. Solid-liquid extraction (SLE) finds extensive use in food processing and environmental remediation, particularly for extracting essential oils from plant materials and removing contaminants from soil samples. Case studies highlight LLE's success in solvent recovery at oil refineries, while SLE demonstrates cost-effective large-scale extraction of antioxidants from natural products.
Future Trends and Innovations in Extraction Technologies
Future trends in liquid-liquid extraction emphasize the integration of membrane technologies and green solvents to enhance selectivity, reduce solvent consumption, and minimize environmental impact. Solid-liquid extraction is advancing through the development of novel adsorbent materials such as molecularly imprinted polymers and nanostructured sorbents that improve extraction efficiency and target specificity. Innovations in automation and real-time process monitoring are expected to drive higher throughput and consistent product quality across both extraction methods.
Partition coefficient
Liquid-liquid extraction relies on the partition coefficient to determine the distribution ratio of a solute between two immiscible liquids, while solid-liquid extraction depends less directly on the partition coefficient and more on solubility and adsorption properties.
Solvent selectivity
Liquid-liquid extraction offers higher solvent selectivity for separating compounds based on differences in solubility between two immiscible liquids, whereas solid-liquid extraction relies on solvent penetration and affinity for solubilizing target components from a solid matrix.
Raffinate phase
The raffinate phase in liquid-liquid extraction typically contains the residual solute concentration after solvent separation, whereas in solid-liquid extraction, the raffinate phase is the solid residue remaining post-extraction with reduced solute content.
Extract phase
The extract phase in liquid-liquid extraction involves transferring solutes between two immiscible liquid solvents, while in solid-liquid extraction it refers to dissolving solutes from a solid matrix into a liquid solvent.
Leaching
Leaching, a solid-liquid extraction process, separates soluble components from a solid matrix using a liquid solvent, whereas liquid-liquid extraction transfers solutes between two immiscible liquid phases based on differential solubility.
Distribution ratio
Liquid-liquid extraction typically achieves higher distribution ratios due to enhanced solute partitioning between immiscible liquid phases, whereas solid-liquid extraction often exhibits lower distribution ratios influenced by solid matrix interactions and limited solute mobility.
Mass transfer resistance
Mass transfer resistance in liquid-liquid extraction is primarily influenced by the interfacial area and diffusion across the liquid-liquid boundary, whereas in solid-liquid extraction, resistance arises mainly from diffusion within the solid matrix and boundary layer around the solid particles.
Countercurrent operation
Countercurrent operation in liquid-liquid extraction enhances separation efficiency by continuously contacting two immiscible liquid phases moving in opposite directions, whereas in solid-liquid extraction, countercurrent flow maximizes solute recovery by sequentially contacting fresh solvent with progressively spent solids.
Solute recovery
Liquid-liquid extraction offers higher solute recovery efficiency for hydrophobic compounds, while solid-liquid extraction is more effective for recovering soluble solids from heterogeneous mixtures.
Phase disengagement
Liquid-liquid extraction offers faster and more efficient phase disengagement compared to solid-liquid extraction, where phase separation is often slower and more complex due to solid matrix interactions.
Liquid-liquid extraction vs Solid-liquid extraction Infographic
 njnir.com
njnir.com