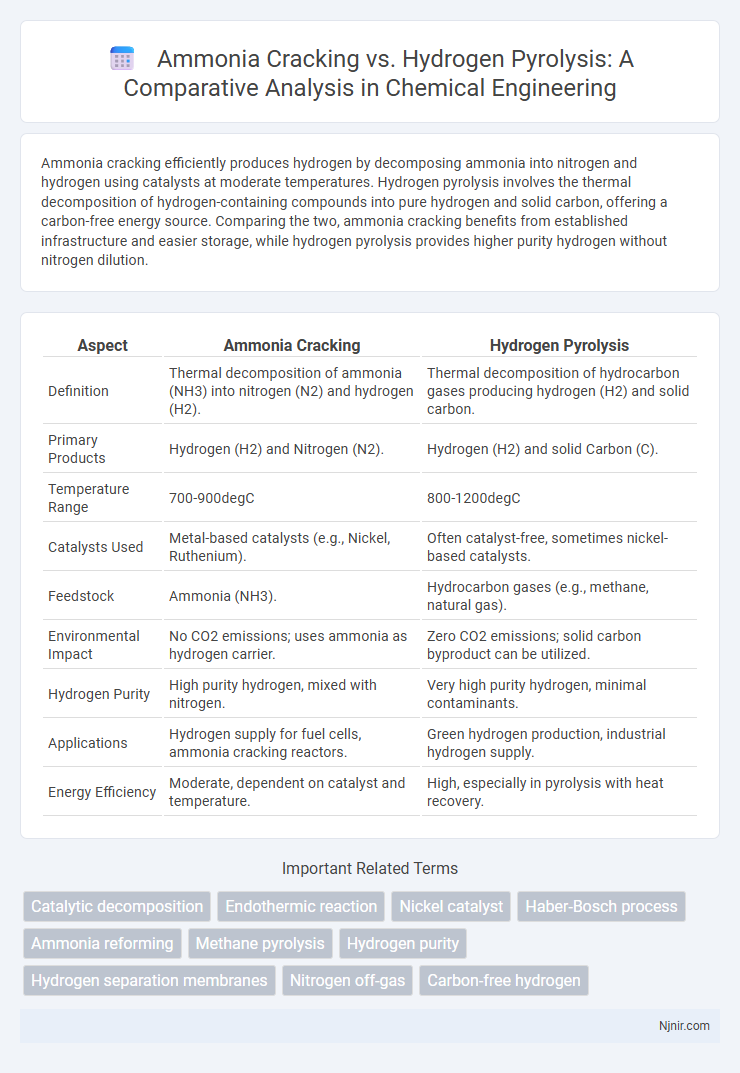
Ammonia cracking efficiently produces hydrogen by decomposing ammonia into nitrogen and hydrogen using catalysts at moderate temperatures. Hydrogen pyrolysis involves the thermal decomposition of hydrogen-containing compounds into pure hydrogen and solid carbon, offering a carbon-free energy source. Comparing the two, ammonia cracking benefits from established infrastructure and easier storage, while hydrogen pyrolysis provides higher purity hydrogen without nitrogen dilution.
Table of Comparison
| Aspect | Ammonia Cracking | Hydrogen Pyrolysis |
|---|---|---|
| Definition | Thermal decomposition of ammonia (NH3) into nitrogen (N2) and hydrogen (H2). | Thermal decomposition of hydrocarbon gases producing hydrogen (H2) and solid carbon. |
| Primary Products | Hydrogen (H2) and Nitrogen (N2). | Hydrogen (H2) and solid Carbon (C). |
| Temperature Range | 700-900degC | 800-1200degC |
| Catalysts Used | Metal-based catalysts (e.g., Nickel, Ruthenium). | Often catalyst-free, sometimes nickel-based catalysts. |
| Feedstock | Ammonia (NH3). | Hydrocarbon gases (e.g., methane, natural gas). |
| Environmental Impact | No CO2 emissions; uses ammonia as hydrogen carrier. | Zero CO2 emissions; solid carbon byproduct can be utilized. |
| Hydrogen Purity | High purity hydrogen, mixed with nitrogen. | Very high purity hydrogen, minimal contaminants. |
| Applications | Hydrogen supply for fuel cells, ammonia cracking reactors. | Green hydrogen production, industrial hydrogen supply. |
| Energy Efficiency | Moderate, dependent on catalyst and temperature. | High, especially in pyrolysis with heat recovery. |
Introduction to Ammonia Cracking and Hydrogen Pyrolysis
Ammonia cracking involves the catalytic decomposition of ammonia (NH3) into nitrogen (N2) and hydrogen (H2), enabling efficient hydrogen release for energy applications and fuel cells. Hydrogen pyrolysis, also known as methane pyrolysis when applied to hydrocarbons, is the thermal decomposition of hydrogen-rich compounds into hydrogen and solid carbon without producing CO2 emissions. Both processes play critical roles in clean hydrogen production, with ammonia cracking offering storage benefits and hydrogen pyrolysis providing carbon-free hydrogen from methane sources.
Chemical Engineering Principles of Ammonia Cracking
Ammonia cracking involves the catalytic decomposition of NH3 into nitrogen and hydrogen gases, relying on catalysts such as nickel, ruthenium, or iron to lower the activation energy and enhance reaction rates. The process follows chemical engineering principles including reaction kinetics, mass transfer, and thermodynamics, with temperature and pressure control crucial for optimizing conversion efficiency and minimizing energy consumption. In contrast, hydrogen pyrolysis focuses on the thermal decomposition of hydrocarbons to produce hydrogen and solid carbon, lacking the specific catalytic pathways central to ammonia cracking.
Fundamentals of Hydrogen Pyrolysis Technology
Hydrogen pyrolysis technology fundamentally involves the thermal decomposition of hydrocarbons into hydrogen gas and solid carbon without combustion, enabling efficient hydrogen production with minimal CO2 emissions. Unlike ammonia cracking, which decomposes NH3 into hydrogen and nitrogen, hydrogen pyrolysis directly processes methane or other hydrocarbons at high temperatures to generate pure hydrogen and recover carbon byproducts. This approach enhances energy efficiency and environmental sustainability by integrating advanced reactor designs and catalysts to optimize hydrogen yield and carbon management.
Reaction Mechanisms: Ammonia Cracking vs Hydrogen Pyrolysis
Ammonia cracking involves breaking NH3 molecules into nitrogen and hydrogen gases using a catalyst, typically nickel or ruthenium, through a heterogeneous catalytic reaction mechanism that includes adsorption, dissociation, and desorption steps. Hydrogen pyrolysis, or methane pyrolysis, decomposes methane (CH4) into solid carbon and molecular hydrogen at high temperatures via thermal cracking without catalysts, where methane molecules undergo bond cleavage into hydrogen radicals and carbon species. The key mechanistic difference lies in ammonia cracking's reliance on surface catalytic interactions and hydrogen pyrolysis's non-catalytic thermal bond dissociation.
Process Conditions and Catalysts Comparison
Ammonia cracking typically operates at temperatures between 700degC and 900degC using nickel-based catalysts to efficiently decompose NH3 into nitrogen and hydrogen, whereas hydrogen pyrolysis requires higher temperatures around 1000degC to break down hydrocarbons with metal catalysts such as molybdenum or nickel. The catalyst choice in ammonia cracking ensures selective ammonia dissociation without coking, while hydrogen pyrolysis catalysts focus on hydrocarbon cracking with resistance to carbon deposition. Process conditions in ammonia cracking favor lower pressure environments to enhance conversion rates, contrasting with hydrogen pyrolysis processes that may operate under varied pressures depending on feedstock and desired hydrogen yield.
Energy Efficiency and Yield Optimization
Ammonia cracking demonstrates high energy efficiency by utilizing catalytic processes to convert ammonia into hydrogen with minimal energy loss, achieving yield rates exceeding 95%. Hydrogen pyrolysis involves decomposing hydrocarbons into hydrogen and solid carbon, presenting challenges in energy optimization due to higher temperature requirements and incomplete conversion rates that typically range between 70-85%. Comparative analyses indicate ammonia cracking is more suitable for scalable hydrogen production with optimized yield and lower overall energy consumption, making it a preferred method in renewable energy applications.
Industrial Applications and Scale-Up Challenges
Ammonia cracking and hydrogen pyrolysis serve as pivotal technologies for hydrogen production in industrial applications, with ammonia cracking gaining traction due to its established infrastructure for ammonia storage and transport. Hydrogen pyrolysis offers the advantage of producing high-purity hydrogen with solid carbon byproduct, making it promising for decarbonizing steel and chemical industries. Scale-up challenges for ammonia cracking involve catalyst optimization and ammonia supply logistics, whereas hydrogen pyrolysis faces issues such as reactor material durability and managing carbon deposition at high production volumes.
Environmental Impact and Emissions Analysis
Ammonia cracking produces hydrogen by breaking down ammonia (NH3) into nitrogen (N2) and hydrogen (H2), resulting in zero carbon dioxide emissions at the point of use, making it an attractive option for decarbonizing hydrogen supply chains. Hydrogen pyrolysis splits methane (CH4) into hydrogen and solid carbon, significantly reducing CO2 emissions compared to steam methane reforming but generating solid carbon that requires sustainable management to avoid environmental harm. Both technologies offer pathways to low-emission hydrogen production, with ammonia cracking reducing greenhouse gases through nitrogen release and hydrogen pyrolysis limiting CO2 but posing challenges in carbon handling and lifecycle emissions.
Economic Considerations and Cost-Benefit Analysis
Ammonia cracking offers a cost advantage due to the existing ammonia supply infrastructure and lower storage costs compared to hydrogen pyrolysis, which demands high-temperature reactors and specialized materials increasing capital expenses. Hydrogen pyrolysis benefits from producing solid carbon as a valuable byproduct, potentially offsetting operational costs, but faces higher energy consumption and maintenance expenses. Economic considerations must balance feedstock availability, energy efficiency, and byproduct value to determine the optimal hydrogen production method.
Future Perspectives in Hydrogen Production Technologies
Hydrogen production via ammonia cracking offers a promising pathway for efficient hydrogen storage and transport, leveraging ammonia's high volumetric energy density and existing infrastructure. Hydrogen pyrolysis presents an emerging technology enabling clean hydrogen generation with solid carbon byproduct, eliminating CO2 emissions and meeting stringent environmental standards. Future advancements emphasize optimizing catalysts, reducing energy consumption, and integrating renewable energy sources to scale both technologies for sustainable hydrogen economy expansion.
Catalytic decomposition
Catalytic decomposition of ammonia achieves efficient hydrogen production with lower energy consumption compared to hydrogen pyrolysis, which typically requires higher temperatures and non-catalytic conditions.
Endothermic reaction
Ammonia cracking and hydrogen pyrolysis are both endothermic reactions where ammonia decomposition requires significant energy input to break N-H bonds, while hydrogen pyrolysis involves energy-intensive dissociation of H2 molecules into atomic hydrogen.
Nickel catalyst
Nickel catalysts enhance ammonia cracking efficiency by lowering activation energy, whereas hydrogen pyrolysis typically requires higher temperatures and different catalysts for effective hydrogen production.
Haber-Bosch process
Ammonia cracking efficiently releases hydrogen produced via the Haber-Bosch process by decomposing NH3, whereas hydrogen pyrolysis directly splits molecular hydrogen without involving ammonia intermediates.
Ammonia reforming
Ammonia reforming efficiently cracks ammonia into hydrogen and nitrogen at elevated temperatures using catalytic reactors, offering a scalable method for hydrogen production compared to hydrogen pyrolysis.
Methane pyrolysis
Methane pyrolysis efficiently produces hydrogen by decomposing methane into solid carbon and hydrogen gas, offering a cleaner alternative to ammonia cracking by avoiding nitrogen-based emissions.
Hydrogen purity
Hydrogen purity from ammonia cracking typically exceeds 99.9% due to efficient catalyst use and selective decomposition, whereas hydrogen pyrolysis achieves ultra-high purity often above 99.99% by avoiding nitrogen contamination inherent in ammonia processing.
Hydrogen separation membranes
Hydrogen separation membranes in ammonia cracking exhibit higher selectivity and durability compared to those used in hydrogen pyrolysis, enabling more efficient hydrogen extraction and reduced energy consumption.
Nitrogen off-gas
Ammonia cracking produces nitrogen-rich off-gas as a primary byproduct, while hydrogen pyrolysis generates minimal nitrogen emissions, making ammonia cracking more significant for managing nitrogen off-gas in hydrogen production.
Carbon-free hydrogen
Ammonia cracking produces carbon-free hydrogen using ammonia decomposition, while hydrogen pyrolysis generates carbon-free hydrogen by splitting methane into hydrogen and solid carbon without CO2 emissions.
Ammonia cracking vs hydrogen pyrolysis Infographic
 njnir.com
njnir.com