Perovskite catalysts exhibit superior thermal stability and oxygen mobility compared to zeolite catalysts, enhancing their performance in high-temperature chemical reactions such as catalytic combustion and oxidation processes. Zeolite catalysts offer remarkable shape selectivity and acidic properties, making them ideal for hydrocarbon cracking and isomerization reactions. The choice between perovskite and zeolite catalysts depends on reaction conditions and specific catalytic requirements, with perovskites favored for oxidative environments and zeolites for acid-catalyzed transformations.
Table of Comparison
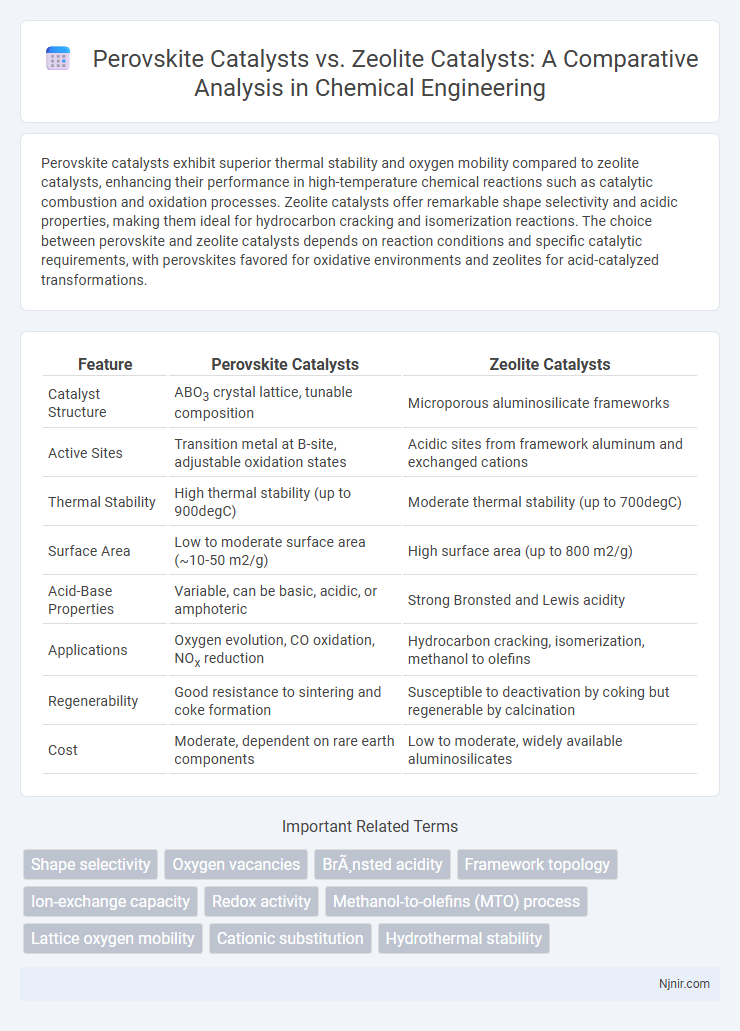
| Feature | Perovskite Catalysts | Zeolite Catalysts |
|---|---|---|
| Catalyst Structure | ABO3 crystal lattice, tunable composition | Microporous aluminosilicate frameworks |
| Active Sites | Transition metal at B-site, adjustable oxidation states | Acidic sites from framework aluminum and exchanged cations |
| Thermal Stability | High thermal stability (up to 900degC) | Moderate thermal stability (up to 700degC) |
| Surface Area | Low to moderate surface area (~10-50 m2/g) | High surface area (up to 800 m2/g) |
| Acid-Base Properties | Variable, can be basic, acidic, or amphoteric | Strong Bronsted and Lewis acidity |
| Applications | Oxygen evolution, CO oxidation, NOx reduction | Hydrocarbon cracking, isomerization, methanol to olefins |
| Regenerability | Good resistance to sintering and coke formation | Susceptible to deactivation by coking but regenerable by calcination |
| Cost | Moderate, dependent on rare earth components | Low to moderate, widely available aluminosilicates |
Introduction to Perovskite and Zeolite Catalysts
Perovskite catalysts feature a distinctive crystal structure characterized by the formula ABX3, where 'A' and 'B' represent metal cations and 'X' is an anion, often oxygen, enabling adjustable electronic and catalytic properties for applications like oxygen evolution and reduction reactions. Zeolite catalysts consist of microporous aluminosilicate frameworks with uniform pore sizes that facilitate shape-selective catalysis, widely used in petrochemical refining and hydrocarbon cracking processes. Both materials are valued for their high surface area and tunable active sites, but perovskites offer enhanced electronic conductivity while zeolites provide exceptional thermal stability and molecular sieving capabilities.
Structural Comparison: Perovskite vs Zeolite Materials
Perovskite catalysts feature a distinctive ABX3 crystal structure with a flexible lattice that allows for diverse elemental substitutions, enhancing catalytic versatility and oxygen ion mobility. Zeolite catalysts possess a rigid tetrahedral framework composed of SiO4 and AlO4 units forming uniform microporous channels and cages, which provide shape-selective catalytic sites and high surface area. The structural differences result in perovskites being more tunable for redox reactions, while zeolites excel in size-selective catalysis due to their well-defined pore architecture.
Synthesis Methods of Perovskite and Zeolite Catalysts
Perovskite catalysts are typically synthesized using methods such as sol-gel processing, solid-state reaction, and co-precipitation to achieve precise control over particle size and composition, enabling tailored catalytic properties. Zeolite catalysts are commonly produced through hydrothermal synthesis involving aluminosilicate gel crystallization under controlled temperature and pH, allowing formation of highly ordered microporous frameworks with specific pore sizes. The choice of synthesis method directly impacts catalytic activity, stability, and selectivity for both perovskite and zeolite materials in various industrial applications.
Catalytic Mechanisms and Active Sites
Perovskite catalysts exhibit unique catalytic mechanisms through their flexible ABO3 crystal structure, enabling oxygen vacancy formation and facile electron transfer at the B-site transition metals, which act as highly active sites for redox reactions. Zeolite catalysts, characterized by their microporous aluminosilicate framework, rely on Bronsted acid sites formed by framework aluminum and proton balancing cations as primary active sites, facilitating shape-selective catalysis via molecular sieving. The distinct active site environments in perovskites promote oxidation and oxygen evolution reactions, whereas zeolites excel in acid-catalyzed hydrocarbon transformations through proton-mediated mechanisms.
Surface Area and Porosity: Impact on Catalytic Performance
Perovskite catalysts typically exhibit lower surface area but higher thermal stability compared to zeolite catalysts, which possess well-defined porous structures with high surface area facilitating enhanced reactant adsorption. The unique pore architecture of zeolites enables selective catalysis through size-exclusion effects, while perovskites offer tunable electronic properties influencing catalytic activity. Surface area and porosity critically impact catalytic performance by controlling reactant diffusion and active site accessibility, with zeolites excelling in shape-selective reactions and perovskites favored for oxidation and reduction processes.
Thermal and Chemical Stability Analysis
Perovskite catalysts exhibit superior thermal stability due to their robust crystal lattice and high tolerance to temperature fluctuations, maintaining structural integrity beyond 800degC. Zeolite catalysts demonstrate excellent chemical stability, particularly in acidic environments, owing to their crystalline aluminosilicate framework that resists deactivation by coking and hydrothermal aging. Comparative analysis reveals perovskites outperform in high-temperature oxidation reactions, while zeolites excel in selective catalysis under moderate thermal conditions.
Application in Industrial Processes
Perovskite catalysts exhibit exceptional activity and thermal stability in industrial processes such as automotive exhaust treatment and methane reforming, offering superior oxygen ion mobility and tunable electronic properties. Zeolite catalysts dominate in fluid catalytic cracking and hydrocarbon isomerization due to their high surface area, shape-selective pores, and strong acid sites enabling precise molecular transformation. Both catalyst types enhance efficiency and selectivity in large-scale chemical manufacturing, with perovskites favored for redox reactions and zeolites excelling in acid-catalyzed processes.
Environmental Impact and Sustainability
Perovskite catalysts demonstrate superior environmental benefits due to their high catalytic efficiency and stability, which reduces energy consumption and waste production in chemical reactions. Zeolite catalysts offer sustainability advantages through their natural abundance, recyclability, and low toxicity, making them environmentally friendly options for large-scale industrial applications. Both catalyst types contribute to green chemistry by enabling cleaner processes and minimizing harmful emissions, with Perovskites excelling in performance and Zeolites emphasizing eco-friendly material properties.
Challenges and Limitations
Perovskite catalysts face challenges such as structural instability under high temperatures and moisture, which limits their long-term durability in industrial applications. Zeolite catalysts are constrained by their microporous structure, causing diffusion limitations for larger molecules and resulting in lower catalytic efficiency in certain reactions. Both materials require optimization to overcome deactivation mechanisms like coking and framework degradation for enhanced performance in catalytic processes.
Future Trends in Catalyst Development
Perovskite catalysts exhibit tunable electronic structures and high oxygen mobility, making them promising for next-generation catalytic technologies in energy conversion and environmental applications. Zeolite catalysts, known for their shape-selectivity and acid-base properties, continue to advance through hierarchical structuring and metal incorporation to enhance catalytic activity and selectivity. Future trends emphasize the integration of perovskite materials with zeolite frameworks to combine the strengths of both catalysts, driving innovations in sustainable chemical processes and green energy production.
Shape selectivity
Perovskite catalysts exhibit tunable crystal structures that enable moderate shape selectivity, while zeolite catalysts provide highly precise shape selectivity due to their uniform microporous frameworks.
Oxygen vacancies
Perovskite catalysts exhibit superior oxygen vacancy concentration compared to zeolite catalysts, enhancing their catalytic activity and oxygen ion mobility for oxidation reactions.
Brønsted acidity
Perovskite catalysts exhibit tunable Bronsted acidity with enhanced proton mobility compared to zeolite catalysts, which possess well-defined, strong Bronsted acid sites confined within their microporous frameworks.
Framework topology
Perovskite catalysts exhibit a flexible, three-dimensional framework topology with corner-sharing octahedra enabling diverse catalytic activities, whereas Zeolite catalysts feature rigid, crystalline aluminosilicate frameworks with well-defined microporous channels that influence molecular selectivity and diffusion.
Ion-exchange capacity
Perovskite catalysts exhibit higher ion-exchange capacity than zeolite catalysts due to their flexible crystal structure and multivalent cation sites, enhancing catalytic performance in ion-exchange applications.
Redox activity
Perovskite catalysts exhibit superior redox activity compared to zeolite catalysts due to their flexible crystal structure and variable oxidation states enabling enhanced oxygen mobility and catalytic efficiency.
Methanol-to-olefins (MTO) process
Perovskite catalysts exhibit higher oxygen mobility and tunable active sites that enhance selectivity and stability in the Methanol-to-olefins (MTO) process compared to traditional zeolite catalysts, which rely on their microporous structure for shape-selective hydrocarbon formation.
Lattice oxygen mobility
Perovskite catalysts exhibit higher lattice oxygen mobility than zeolite catalysts, enhancing their efficacy in redox reactions and catalytic oxidation processes.
Cationic substitution
Cationic substitution in perovskite catalysts enhances their tunable electronic structure and catalytic activity, offering superior selectivity and stability compared to zeolite catalysts.
Hydrothermal stability
Perovskite catalysts exhibit superior hydrothermal stability compared to zeolite catalysts, maintaining structural integrity and catalytic performance under high-temperature steam conditions.
Perovskite catalysts vs Zeolite catalysts Infographic
 njnir.com
njnir.com