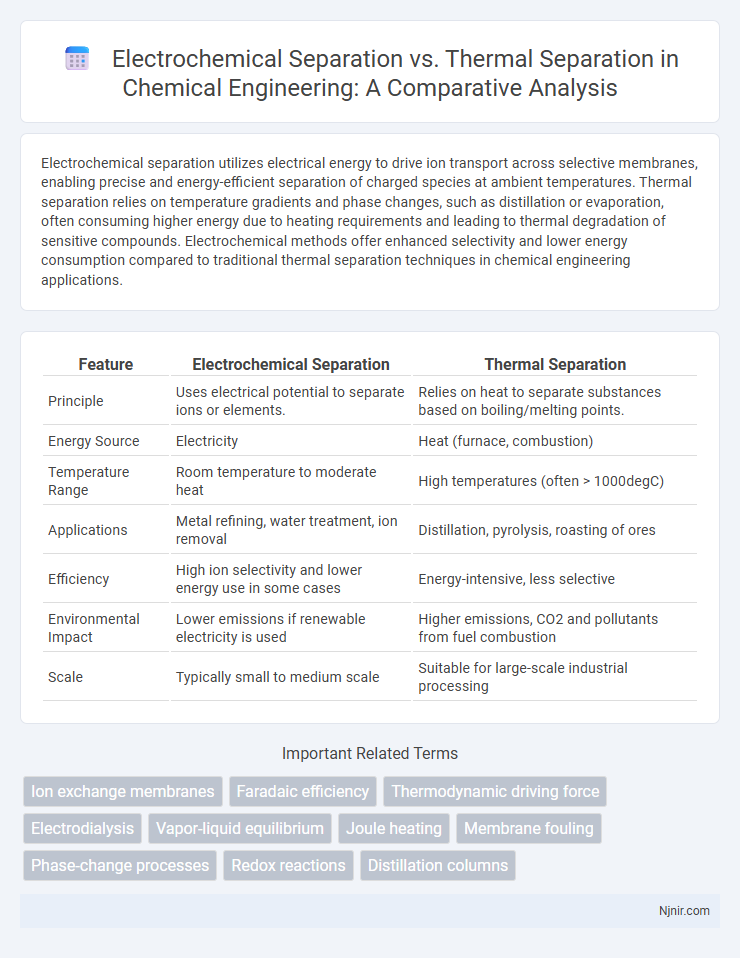
Electrochemical separation utilizes electrical energy to drive ion transport across selective membranes, enabling precise and energy-efficient separation of charged species at ambient temperatures. Thermal separation relies on temperature gradients and phase changes, such as distillation or evaporation, often consuming higher energy due to heating requirements and leading to thermal degradation of sensitive compounds. Electrochemical methods offer enhanced selectivity and lower energy consumption compared to traditional thermal separation techniques in chemical engineering applications.
Table of Comparison
| Feature | Electrochemical Separation | Thermal Separation |
|---|---|---|
| Principle | Uses electrical potential to separate ions or elements. | Relies on heat to separate substances based on boiling/melting points. |
| Energy Source | Electricity | Heat (furnace, combustion) |
| Temperature Range | Room temperature to moderate heat | High temperatures (often > 1000degC) |
| Applications | Metal refining, water treatment, ion removal | Distillation, pyrolysis, roasting of ores |
| Efficiency | High ion selectivity and lower energy use in some cases | Energy-intensive, less selective |
| Environmental Impact | Lower emissions if renewable electricity is used | Higher emissions, CO2 and pollutants from fuel combustion |
| Scale | Typically small to medium scale | Suitable for large-scale industrial processing |
Introduction to Electrochemical and Thermal Separation
Electrochemical separation leverages electrical potential to drive ion-selective transport across membranes, enabling efficient extraction and purification of metals and salts. Thermal separation relies on temperature gradients and phase changes, such as distillation or evaporation, to segregate components based on differing boiling points or vapor pressures. Both methods address material recovery with distinct energy inputs and selectivity, impacting their suitability for applications like wastewater treatment or metal refining.
Principles of Electrochemical Separation
Electrochemical separation relies on selective ion transport through membranes driven by an electric potential difference, enabling precise separation of charged species based on their electrochemical properties. This method utilizes redox reactions or ion exchange processes at electrodes to facilitate the migration or deposition of target ions, providing energy-efficient and highly selective separation compared to thermal methods. Electrochemical techniques offer advantages such as lower operating temperatures and reduced environmental impact, making them suitable for applications like desalination, metal recovery, and wastewater treatment.
Fundamentals of Thermal Separation Techniques
Thermal separation techniques, such as distillation, evaporation, and membrane separation, rely on differences in physical properties like boiling points and vapor pressures to achieve component separation by applying heat. These processes exploit phase changes induced by temperature gradients to separate mixtures into their constituent components, often requiring significant energy input due to latent heat requirements. Understanding thermodynamic principles, including phase equilibria and heat transfer mechanisms, is essential for optimizing thermal separation efficiency and designing industrial systems.
Energy Efficiency Comparison
Electrochemical separation processes typically exhibit higher energy efficiency compared to thermal separation techniques due to their ability to operate at lower temperatures and directly utilize electrical energy for ion or molecule transport. Thermal separation methods, such as distillation or evaporation, demand significant heat input often sourced from fossil fuels, resulting in substantial energy losses and higher carbon emissions. Advances in electrochemical technologies, including membrane-based electrolysis and electrodialysis, further enhance energy savings by enabling selective separation with minimal thermal input.
Material Selectivity and Purity
Electrochemical separation offers high material selectivity through precise control of voltage and current, enabling targeted ion removal with minimal cross-contamination, resulting in superior purity levels. Thermal separation relies on differences in boiling points or phase changes, which can lead to co-distillation of impurities and lower selectivity due to overlapping thermal properties of materials. Consequently, electrochemical methods typically provide enhanced purity and selective extraction, especially for complex or thermally sensitive mixtures.
Technological Advancements and Equipment
Electrochemical separation leverages advancements in membrane technology and electrode materials, enabling higher selectivity and energy efficiency in processes such as ion exchange and electrolysis, with equipment like ion-selective membranes and advanced electrochemical reactors. Thermal separation benefits from innovations in heat integration, improved insulation, and high-performance distillation columns or crystallizers, optimizing energy consumption and throughput. Both technologies increasingly incorporate automation and process control systems to enhance precision and scalability in industrial separation applications.
Environmental Impact Assessment
Electrochemical separation processes typically offer lower energy consumption and reduced greenhouse gas emissions compared to traditional thermal separation methods, making them more environmentally sustainable. Thermal separation relies heavily on high temperatures, which result in significant carbon dioxide emissions and higher fossil fuel demand, exacerbating climate change impacts. Environmental Impact Assessments (EIA) highlight that electrochemical techniques produce less hazardous waste and minimize air pollutants, contributing to improved air quality and lower ecological footprints in industrial applications.
Cost Analysis and Economic Considerations
Electrochemical separation typically offers lower operational costs due to its higher energy efficiency and reduced thermal losses compared to thermal separation methods, but initial capital expenses for specialized equipment can be significant. Thermal separation processes often involve higher energy consumption and maintenance costs, especially in large-scale industrial applications, resulting in greater long-term operational expenses. Economic considerations must balance the upfront investment and running costs, with electrochemical methods becoming increasingly cost-competitive in applications requiring energy-efficient and environmentally friendly separation solutions.
Industrial Applications and Case Studies
Electrochemical separation techniques, such as electrodialysis and electrolysis, offer energy-efficient solutions for industries like wastewater treatment and metal recovery, showcasing high selectivity and lower thermal degradation compared to thermal separation. Thermal separation processes, including distillation and evaporation, dominate chemical manufacturing and petrochemical plants due to their scalability and ability to handle high-volume separations despite higher energy consumption. Case studies reveal that electrochemical methods reduce operational costs in brine desalination projects, while thermal techniques remain essential for purifying chemical feedstocks and biofuel production.
Future Trends in Separation Technologies
Electrochemical separation technologies are advancing with innovations in membrane materials and energy-efficient electrolysis, offering precise ion-selective extraction for water treatment and resource recovery. Thermal separation methods are evolving through integration with renewable energy sources and enhanced heat recovery systems, reducing energy consumption in processes like distillation and evaporation. Future trends prioritize hybrid systems combining electrochemical and thermal approaches for optimized sustainability and performance in industrial separation applications.
Ion exchange membranes
Electrochemical separation using ion exchange membranes offers higher selectivity and energy efficiency compared to thermal separation methods by enabling targeted ion transport under controlled electrical potentials.
Faradaic efficiency
Electrochemical separation achieves higher Faradaic efficiency compared to thermal separation by directly converting electrical energy into chemical changes, minimizing energy loss and enhancing selective ion transport.
Thermodynamic driving force
Electrochemical separation utilizes electrical potential as the thermodynamic driving force, whereas thermal separation relies on temperature gradients to achieve material separation.
Electrodialysis
Electrodialysis, an electrochemical separation process, offers energy-efficient ion removal with higher selectivity and lower environmental impact compared to traditional thermal separation methods such as distillation or evaporation.
Vapor-liquid equilibrium
Electrochemical separation leverages ion-selective transport mechanisms distinct from thermal separation, which relies on vapor-liquid equilibrium properties such as temperature and pressure to achieve component separation.
Joule heating
Electrochemical separation leverages selective ion transport with minimal Joule heating compared to thermal separation, which relies on high-temperature gradients causing significant Joule heating and energy loss.
Membrane fouling
Electrochemical separation reduces membrane fouling by enabling selective ion transport through electrically driven processes, whereas thermal separation often accelerates fouling due to high temperatures and concentration polarization effects on membrane surfaces.
Phase-change processes
Electrochemical separation utilizes ion-selective membranes and electrical potential to achieve precise phase-change processes, whereas thermal separation relies on temperature-induced phase transitions like evaporation or condensation to separate components.
Redox reactions
Electrochemical separation leverages precise redox reactions at electrode interfaces to selectively transfer ions or electrons, offering higher energy efficiency and specificity compared to thermal separation methods that rely on bulk phase changes driven by heat.
Distillation columns
Electrochemical separation offers higher energy efficiency and selective ion removal compared to thermal separation in distillation columns, which rely on heat-intensive phase changes to separate mixtures.
Electrochemical separation vs Thermal separation Infographic
 njnir.com
njnir.com