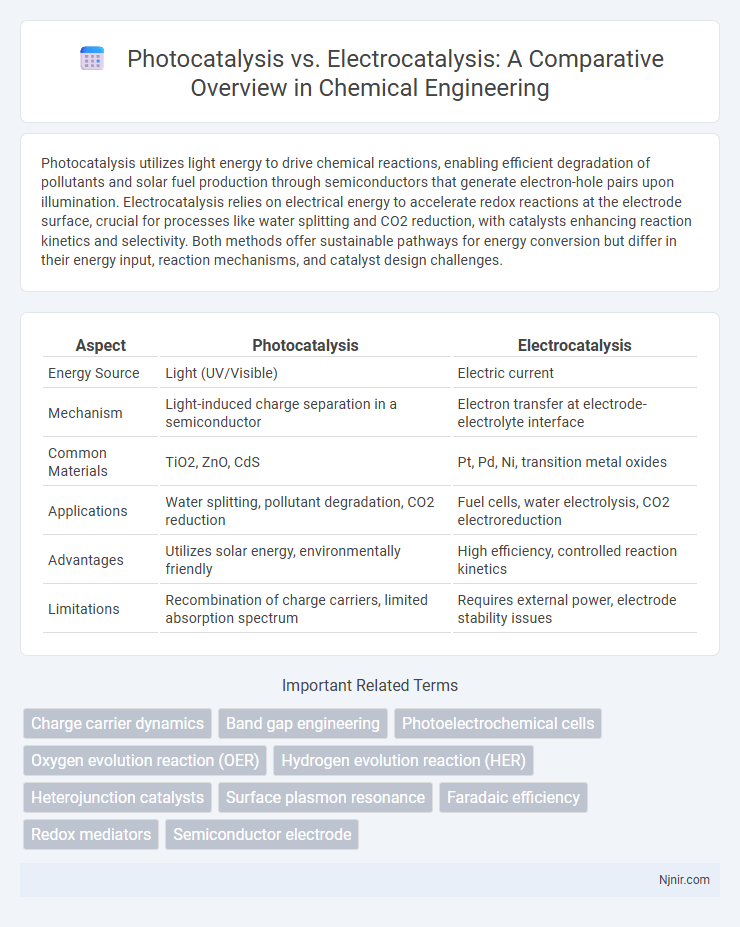
Photocatalysis utilizes light energy to drive chemical reactions, enabling efficient degradation of pollutants and solar fuel production through semiconductors that generate electron-hole pairs upon illumination. Electrocatalysis relies on electrical energy to accelerate redox reactions at the electrode surface, crucial for processes like water splitting and CO2 reduction, with catalysts enhancing reaction kinetics and selectivity. Both methods offer sustainable pathways for energy conversion but differ in their energy input, reaction mechanisms, and catalyst design challenges.
Table of Comparison
| Aspect | Photocatalysis | Electrocatalysis |
|---|---|---|
| Energy Source | Light (UV/Visible) | Electric current |
| Mechanism | Light-induced charge separation in a semiconductor | Electron transfer at electrode-electrolyte interface |
| Common Materials | TiO2, ZnO, CdS | Pt, Pd, Ni, transition metal oxides |
| Applications | Water splitting, pollutant degradation, CO2 reduction | Fuel cells, water electrolysis, CO2 electroreduction |
| Advantages | Utilizes solar energy, environmentally friendly | High efficiency, controlled reaction kinetics |
| Limitations | Recombination of charge carriers, limited absorption spectrum | Requires external power, electrode stability issues |
Introduction to Photocatalysis and Electrocatalysis
Photocatalysis harnesses light energy to drive chemical reactions via semiconductor materials that generate electron-hole pairs, facilitating processes like pollutant degradation and water splitting. Electrocatalysis involves the acceleration of electrochemical reactions on electrode surfaces, enhancing efficiency in energy conversion systems such as fuel cells and electrolyzers. Both methods play critical roles in sustainable energy and environmental applications by enabling catalytic processes under mild conditions.
Fundamental Principles of Photocatalysis
Photocatalysis relies on semiconductor materials that absorb light to generate electron-hole pairs, facilitating redox reactions on the catalyst surface. The fundamental principle involves photoexcitation, charge carrier separation, and migration to active sites where reactants undergo chemical transformation. This process harnesses solar energy for environmental remediation and renewable fuel production, differentiating it from electrocatalysis, which depends on externally applied electrical potential.
Core Mechanisms in Electrocatalysis
Electrocatalysis involves the acceleration of electrochemical reactions at the electrode-electrolyte interface through catalyst materials that facilitate electron transfer and lower activation energy barriers. Key mechanisms include adsorption of reactants on the catalyst surface, electron-proton transfer steps, and the formation of reaction intermediates which drive processes like hydrogen evolution and oxygen reduction. Charge transfer kinetics and surface catalytic sites are critical factors influencing electrocatalytic efficiency and selectivity.
Comparative Analysis: Photocatalysis vs. Electrocatalysis
Photocatalysis uses light energy to accelerate chemical reactions, primarily involving semiconductor materials that generate electron-hole pairs under illumination, enabling processes like pollutant degradation and water splitting. Electrocatalysis utilizes electrical energy to drive reactions at electrode surfaces, often employing metal catalysts such as platinum or nickel to enhance reaction rates in applications like fuel cells and electrolysis. Compared to electrocatalysis, photocatalysis offers the advantage of directly harnessing solar energy but generally exhibits lower reaction efficiencies and slower kinetics, while electrocatalysis provides more controllable reaction conditions and higher catalytic activity under standard laboratory and industrial settings.
Materials and Catalyst Design Strategies
Photocatalysis materials often emphasize semiconductors such as TiO2, ZnO, and CdS, engineered to optimize light absorption and charge separation through doping, heterojunctions, and surface modification. Electrocatalysis catalyst design prioritizes metals and alloys like Pt, RuO2, and Ni-based compounds, focusing on enhancing active site density, conductivity, and stability via nanostructuring and support integration. Both fields increasingly exploit computational modeling and high-throughput screening to tailor catalyst electronic properties and surface chemistry for improved reaction kinetics and selectivity.
Efficiency Metrics and Performance Indicators
Photocatalysis and electrocatalysis efficiency metrics are primarily evaluated by quantum efficiency and Faradaic efficiency, respectively, which quantify the efficacy of photon-to-chemical energy conversion and electron utilization in catalytic reactions. Photocatalytic performance indicators include absorption spectrum range, charge carrier lifetime, and apparent quantum yield, reflecting light harvesting and charge separation capabilities. Electrocatalysis performance is often gauged using overpotential, current density, turnover frequency, and stability under operational conditions, highlighting catalyst activity and durability in electrochemical processes.
Key Applications in Chemical Engineering
Photocatalysis plays a critical role in environmental remediation, enabling the degradation of organic pollutants under visible light through semiconductor catalysts like TiO2. Electrocatalysis is essential in energy conversion technologies, particularly in fuel cells and electrolyzers, where efficient electrocatalysts such as platinum and nickel-based materials drive reactions like hydrogen evolution and oxygen reduction. Both approaches are pivotal in green chemical engineering, facilitating sustainable processes for water splitting, CO2 reduction, and waste treatment.
Environmental and Energy Implications
Photocatalysis harnesses light energy to drive chemical reactions that degrade pollutants and produce clean fuels like hydrogen, offering a sustainable approach for environmental remediation and renewable energy generation. Electrocatalysis uses electrical energy to accelerate redox reactions, enabling efficient conversion of CO2 into hydrocarbons and water splitting for hydrogen production, essential for reducing carbon emissions and advancing green energy technologies. Both technologies contribute significantly to environmental protection and energy sustainability by enabling low-emission processes and promoting the use of abundant resources like sunlight and electricity from renewable sources.
Challenges and Limitations in Each Approach
Photocatalysis faces challenges such as low solar-to-fuel conversion efficiency and the rapid recombination of photogenerated electron-hole pairs, which limit its overall performance. Electrocatalysis often struggles with high overpotentials and catalyst degradation during prolonged operation, reducing its energy efficiency and durability. Both approaches require the development of highly active, stable, and cost-effective catalysts to overcome these intrinsic limitations for practical applications.
Future Trends and Research Directions
Emerging research in photocatalysis emphasizes the development of highly efficient visible-light-responsive materials and hybrid systems to enhance solar energy conversion and pollutant degradation. Electrocatalysis advancements focus on designing nanoscale catalysts with tailored active sites to improve reaction kinetics and selectivity in energy storage and conversion devices like fuel cells and electrolyzers. Future trends highlight the integration of artificial intelligence for catalyst discovery and the exploration of sustainable, earth-abundant materials for scalable applications.
Charge carrier dynamics
Photocatalysis relies on light-induced charge carrier generation and separation to drive chemical reactions, while electrocatalysis depends on electrode-induced charge transfer dynamics for enhanced catalytic activity.
Band gap engineering
Band gap engineering in photocatalysis tailors semiconductor energy levels to enhance light absorption and charge separation, while in electrocatalysis it optimizes electronic structures to improve catalytic activity and electron transfer efficiency.
Photoelectrochemical cells
Photoelectrochemical cells integrate photocatalysis and electrocatalysis to efficiently convert solar energy into chemical fuels by driving redox reactions at semiconductor-electrode interfaces.
Oxygen evolution reaction (OER)
Photocatalysis and electrocatalysis for the Oxygen Evolution Reaction (OER) differ fundamentally in their energy inputs, with photocatalysis harnessing solar energy to drive water oxidation on semiconductor surfaces, while electrocatalysis relies on electrical energy to facilitate OER on electrode catalysts, emphasizing the material properties and charge transfer efficiencies for improved catalytic performance.
Hydrogen evolution reaction (HER)
Photocatalysis utilizes light energy to drive the hydrogen evolution reaction (HER) by generating charge carriers on semiconductor surfaces, while electrocatalysis employs an external electric potential to facilitate HER through efficient electron transfer on catalytic electrodes.
Heterojunction catalysts
Heterojunction catalysts enhance charge separation and accelerate reaction rates in photocatalysis and electrocatalysis by combining materials with complementary band structures for improved hydrogen evolution efficiency.
Surface plasmon resonance
Surface plasmon resonance enhances photocatalysis by boosting light absorption and charge separation, while in electrocatalysis it improves electron transfer efficiency at the catalyst surface.
Faradaic efficiency
Photocatalysis often exhibits lower Faradaic efficiency compared to electrocatalysis due to charge recombination losses, while electrocatalysis achieves higher Faradaic efficiency by directly driving electron transfer with controlled applied potentials.
Redox mediators
Redox mediators in photocatalysis enhance charge transfer efficiency by facilitating electron-hole separation under light irradiation, while in electrocatalysis, they improve reaction kinetics by shuttling electrons between electrodes and reactants.
Semiconductor electrode
Semiconductor electrodes in photocatalysis harness light energy to drive chemical reactions by generating electron-hole pairs, while in electrocatalysis they facilitate electron transfer processes under applied voltage, each optimizing catalytic efficiency according to their distinct charge carrier dynamics.
Photocatalysis vs Electrocatalysis Infographic
 njnir.com
njnir.com