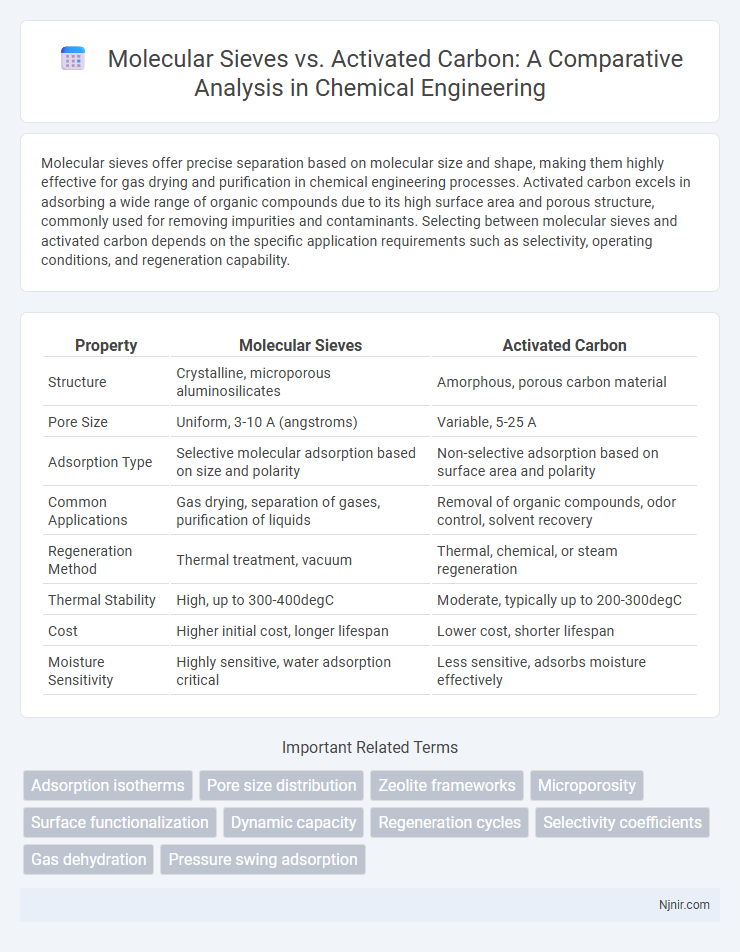
Molecular sieves offer precise separation based on molecular size and shape, making them highly effective for gas drying and purification in chemical engineering processes. Activated carbon excels in adsorbing a wide range of organic compounds due to its high surface area and porous structure, commonly used for removing impurities and contaminants. Selecting between molecular sieves and activated carbon depends on the specific application requirements such as selectivity, operating conditions, and regeneration capability.
Table of Comparison
| Property | Molecular Sieves | Activated Carbon |
|---|---|---|
| Structure | Crystalline, microporous aluminosilicates | Amorphous, porous carbon material |
| Pore Size | Uniform, 3-10 A (angstroms) | Variable, 5-25 A |
| Adsorption Type | Selective molecular adsorption based on size and polarity | Non-selective adsorption based on surface area and polarity |
| Common Applications | Gas drying, separation of gases, purification of liquids | Removal of organic compounds, odor control, solvent recovery |
| Regeneration Method | Thermal treatment, vacuum | Thermal, chemical, or steam regeneration |
| Thermal Stability | High, up to 300-400degC | Moderate, typically up to 200-300degC |
| Cost | Higher initial cost, longer lifespan | Lower cost, shorter lifespan |
| Moisture Sensitivity | Highly sensitive, water adsorption critical | Less sensitive, adsorbs moisture effectively |
Introduction to Molecular Sieves and Activated Carbon
Molecular sieves are crystalline aluminosilicates with uniform pore sizes that selectively adsorb molecules based on size and shape, making them ideal for gas drying and separation processes. Activated carbon consists of a highly porous carbon structure with a large surface area that adsorbs organic compounds, odors, and impurities from liquids and gases. Both materials are essential in industrial applications, with molecular sieves excelling in precision adsorption and activated carbon offering broad-spectrum adsorption capabilities.
Chemical Structure and Composition
Molecular sieves are crystalline aluminosilicates composed of a porous three-dimensional framework of silicon, aluminum, and oxygen atoms, forming uniform micropores that selectively adsorb molecules based on size and polarity. Activated carbon consists of a predominantly amorphous carbon matrix with a high surface area, featuring a complex network of micropores, mesopores, and macropores, and is rich in surface functional groups such as hydroxyl, carbonyl, and carboxyl groups. The rigid, well-defined pore structure of molecular sieves provides precise molecular selectivity, while activated carbon's diverse porous architecture and surface chemistry enable broad-spectrum adsorption through physical and chemical interactions.
Mechanisms of Adsorption
Molecular sieves utilize a crystalline aluminosilicate framework with uniform pore sizes to selectively adsorb molecules based on size exclusion and polarity differences, enabling precise separation of gases and liquids. Activated carbon relies on a highly porous amorphous structure with large surface area and varying pore sizes that adsorb molecules primarily through van der Waals forces and surface interactions. The adsorption mechanism in molecular sieves is predominantly physical and steric, while activated carbon combines physical adsorption with chemical adsorption depending on functional groups on its surface.
Pore Size and Surface Area Comparison
Molecular sieves possess uniform pore sizes typically ranging from 3 to 10 angstroms, enabling precise molecular separation, whereas activated carbon exhibits a broader pore size distribution from micropores (~7 angstroms) to mesopores and macropores, enhancing adsorption versatility. The surface area of activated carbon generally exceeds 1000 m2/g due to its highly porous structure, while molecular sieves offer surface areas around 600 to 800 m2/g optimized for selective adsorption. This distinction in pore size uniformity and surface area directly influences their applications in gas purification, drying, and catalyst support.
Selectivity and Efficiency in Separation Processes
Molecular sieves exhibit high selectivity in separation processes due to their uniform pore size, enabling precise adsorption of specific molecules based on size and polarity. Activated carbon offers broad-spectrum adsorption capacity with high surface area but lacks the precise molecular discrimination of molecular sieves, making it less efficient for targeted separations. Efficiency-wise, molecular sieves achieve faster and more consistent separations in gas and liquid phase applications, while activated carbon is preferred for removing a wide range of organic contaminants in diverse industrial settings.
Regeneration and Reusability
Molecular sieves exhibit high regeneration efficiency through thermal activation, allowing effective removal of adsorbed molecules and restoring adsorption capacity multiple times without significant loss. Activated carbon offers versatile regeneration methods, including thermal, chemical, and steam treatments, though its adsorption efficiency may decline after repeated cycles due to pore structure degradation. Both materials provide reusable adsorption solutions, but molecular sieves maintain consistent performance over extended regeneration cycles, making them preferable for applications demanding high durability.
Applications in Chemical Engineering Industries
Molecular sieves are widely used in chemical engineering industries for gas drying, purification, and separation processes due to their uniform pore size and high adsorption capacity for specific molecules. Activated carbon finds extensive applications in solvent recovery, wastewater treatment, and removal of organic contaminants because of its large surface area and strong adsorption of non-polar compounds. Both materials play critical roles in catalysis, gas purification, and environmental control, with molecular sieves preferred for precision separation and activated carbon favored for broad-spectrum adsorption.
Performance Under Varying Conditions
Molecular sieves maintain high adsorption efficiency across a broad range of temperatures and pressures due to their uniform pore structure, making them ideal for precise gas drying and separation applications. Activated carbon offers versatile adsorption capabilities with strong affinity for organic compounds and impurities, but its performance can decline under high humidity or variable temperature conditions. The choice between molecular sieves and activated carbon depends on the specific environmental factors and target contaminants involved in the separation or purification process.
Environmental and Economic Considerations
Molecular sieves provide superior selectivity and regeneration efficiency, resulting in lower long-term operational costs and reduced environmental impact compared to activated carbon, which tends to have higher disposal requirements and lower adsorption capacity. The energy consumption for regenerating molecular sieves is typically less intensive, translating to decreased greenhouse gas emissions and improved sustainability credentials. Economic evaluations frequently favor molecular sieves for large-scale industrial applications due to their durability and consistent performance, whereas activated carbon may be more cost-effective for smaller or less frequent use scenarios despite increased waste management expenses.
Summary: Choosing Between Molecular Sieves and Activated Carbon
Molecular sieves excel in separating gases and drying applications due to their uniform pore size and high adsorption capacity for specific molecules. Activated carbon offers superior performance in removing organic compounds, odors, and impurities from liquids and gases through its high surface area and diverse pore structure. Selecting between molecular sieves and activated carbon depends on the target molecules, process requirements, and specificity of adsorption needed for optimal purification or separation.
Adsorption isotherms
Molecular sieves exhibit highly selective adsorption isotherms characterized by sharp uptake at specific pressures due to uniform pore size, whereas activated carbon shows broader, less defined isotherms reflecting heterogeneous pore structures and varying adsorption energies.
Pore size distribution
Molecular sieves have uniform pore size distribution typically around 3-10 angstroms, enabling precise separation of molecules, whereas activated carbon exhibits a broad pore size distribution from micropores (<2 nm) to mesopores (2-50 nm), facilitating adsorption of a wide range of molecular sizes.
Zeolite frameworks
Zeolite frameworks in molecular sieves provide highly selective adsorption and superior thermal stability compared to the broad-spectrum adsorption capabilities of activated carbon.
Microporosity
Molecular sieves possess highly uniform micropores sized between 3-10 A, enabling selective adsorption based on molecule size, whereas activated carbon exhibits a broader range of micropore sizes with less uniformity, affecting its adsorption specificity and capacity.
Surface functionalization
Molecular sieves exhibit precise surface functionalization through uniform pore sizes and selective adsorption sites, whereas activated carbon features heterogeneous surface functional groups enhancing broad-spectrum adsorption and catalytic properties.
Dynamic capacity
Molecular sieves exhibit higher dynamic capacity for gas adsorption compared to activated carbon due to their uniform pore size and selective adsorption properties.
Regeneration cycles
Molecular sieves maintain high adsorption efficiency through over 50 regeneration cycles, whereas activated carbon typically degrades after 10-20 cycles due to pore structure collapse and reduced adsorption capacity.
Selectivity coefficients
Molecular sieves exhibit higher selectivity coefficients than activated carbon due to their uniform pore size distribution and tailored adsorption properties for specific gas molecules.
Gas dehydration
Molecular sieves offer superior gas dehydration efficiency and selectivity compared to activated carbon due to their uniform pore size and higher adsorption capacity for water molecules.
Pressure swing adsorption
Pressure swing adsorption using molecular sieves offers higher gas purity and selectivity compared to activated carbon due to its superior adsorption capacity for specific gas molecules under variable pressure conditions.
Molecular sieves vs Activated carbon Infographic
 njnir.com
njnir.com