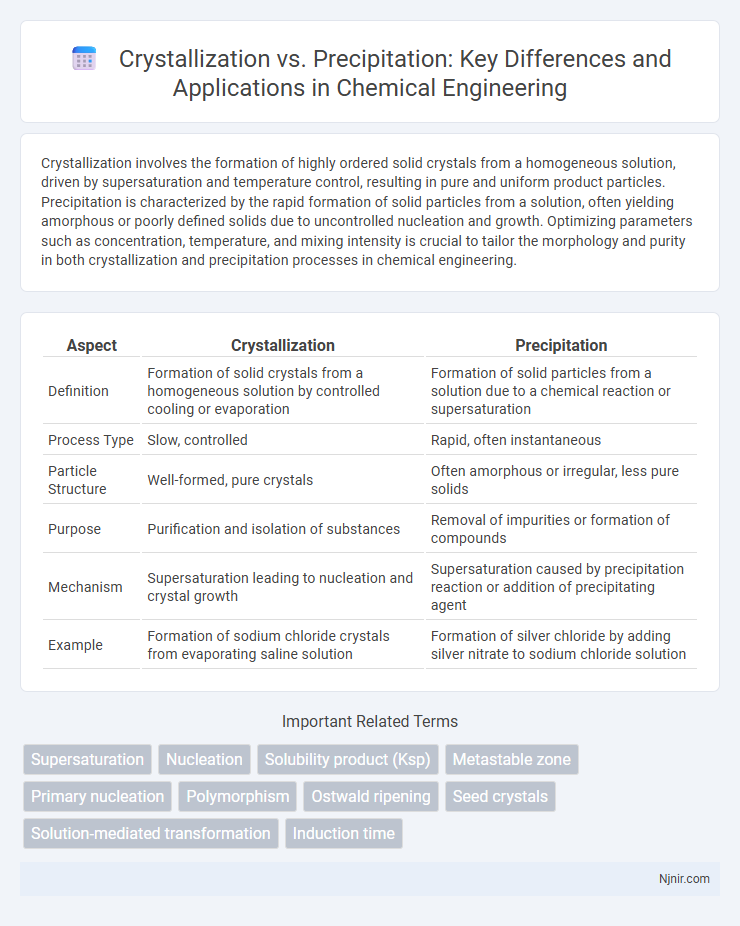
Crystallization involves the formation of highly ordered solid crystals from a homogeneous solution, driven by supersaturation and temperature control, resulting in pure and uniform product particles. Precipitation is characterized by the rapid formation of solid particles from a solution, often yielding amorphous or poorly defined solids due to uncontrolled nucleation and growth. Optimizing parameters such as concentration, temperature, and mixing intensity is crucial to tailor the morphology and purity in both crystallization and precipitation processes in chemical engineering.
Table of Comparison
| Aspect | Crystallization | Precipitation |
|---|---|---|
| Definition | Formation of solid crystals from a homogeneous solution by controlled cooling or evaporation | Formation of solid particles from a solution due to a chemical reaction or supersaturation |
| Process Type | Slow, controlled | Rapid, often instantaneous |
| Particle Structure | Well-formed, pure crystals | Often amorphous or irregular, less pure solids |
| Purpose | Purification and isolation of substances | Removal of impurities or formation of compounds |
| Mechanism | Supersaturation leading to nucleation and crystal growth | Supersaturation caused by precipitation reaction or addition of precipitating agent |
| Example | Formation of sodium chloride crystals from evaporating saline solution | Formation of silver chloride by adding silver nitrate to sodium chloride solution |
Introduction to Crystallization and Precipitation
Crystallization and precipitation are fundamental processes in chemical and physical systems that involve the formation of solid particles from a solution or melt. Crystallization refers to the formation of highly ordered solid crystals with a defined geometric shape through nucleation and growth, driven by supersaturation or temperature changes. Precipitation, in contrast, typically produces amorphous or poorly ordered solids as a consequence of exceeding solubility limits, often occurring rapidly without the development of a crystal lattice.
Fundamental Principles of Crystallization
Crystallization is a purification process where a solid forms from a solution, melt, or gas phase, characterized by the orderly molecular arrangement into a crystalline lattice. This process depends on factors such as supersaturation, temperature control, and nucleation rate, which influence crystal size, purity, and morphology. Unlike precipitation, crystallization emphasizes slow and controlled particle formation to yield high-purity crystals with defined structural properties.
Fundamental Principles of Precipitation
Precipitation involves the formation of solid particles within a solution when the concentration of dissolved ions exceeds their solubility product, leading to nucleation and particle growth. Unlike crystallization, which emphasizes the orderly arrangement of atoms into a crystal lattice, precipitation primarily depends on supersaturation and ionic interactions. Factors such as temperature, concentration, and ionic strength directly influence the kinetics and morphology of the precipitate formed during the precipitation process.
Key Differences Between Crystallization and Precipitation
Crystallization involves the formation of solid crystals from a homogeneous solution, where molecules or ions arrange into an orderly, repeating lattice structure, whereas precipitation typically refers to the rapid formation of insoluble solid particles that may lack a defined crystal structure. Crystallization is a controlled process influenced by factors such as temperature, concentration, and solvent properties, often resulting in high-purity products, while precipitation often occurs spontaneously due to supersaturation or chemical reactions, producing amorphous or less-pure solids. The key differences lie in the kinetics, structural order, and purity of the solids formed, with crystallization favoring slow, orderly growth and precipitation favoring rapid solid formation.
Thermodynamic Aspects in Both Processes
Crystallization and precipitation are governed by thermodynamic principles involving supersaturation, solubility, and nucleation energy barriers. Crystallization requires a controlled reduction in solute solubility, favoring orderly lattice formation with lower Gibbs free energy, while precipitation often occurs through rapid supersaturation leading to amorphous or less ordered solid phases. Thermodynamic driving forces in crystallization emphasize equilibrium and energy minimization, contrasting with the kinetically dominated, unstable conditions typical of precipitation.
Kinetics of Crystal Formation and Precipitate Generation
Crystallization involves the organized and gradual arrangement of molecules into a well-defined lattice, typically controlled by supersaturation and temperature, resulting in slower kinetic rates compared to precipitation. Precipitation occurs rapidly due to the sudden formation of insoluble particles from a solution, driven by quick nucleation and crystal growth processes under high supersaturation conditions. The kinetics of crystal formation during crystallization favor controlled growth and purity, whereas precipitation emphasizes rapid particle generation with less structural order.
Equipment and Techniques Used in Crystallization vs Precipitation
Crystallization typically employs equipment such as crystallizers, cooling or evaporative vessels, and controlled stirring systems to facilitate crystal formation under precise temperature and supersaturation conditions. Precipitation relies on reactors, mixing tanks, and sometimes centrifuges or filtration units to induce rapid solid particle formation from a solution through chemical reactions or changes in solubility. Techniques in crystallization focus on controlled nucleation and crystal growth for purity and size control, whereas precipitation techniques emphasize rapid nucleation and separation of solids from liquids.
Industrial Applications of Crystallization and Precipitation
Industrial crystallization is widely employed for purifying chemicals, producing pharmaceuticals, and fabricating high-purity salts by controlling solubility and supersaturation conditions to form uniform crystal structures. Precipitation finds extensive use in wastewater treatment, extracting metals from ores, and manufacturing pigments, leveraging rapid solid formation from solution to remove contaminants or isolate compounds. Both processes optimize material recovery and product quality but differ in crystal morphology control and purity levels critical to applications like semiconductor fabrication and specialty chemical production.
Challenges and Limitations in Each Process
Crystallization faces challenges such as controlling nucleation rates and crystal purity, which directly impact product quality and yield consistency. Precipitation often struggles with particle size distribution and sedimentation issues, leading to difficulties in separation and filtration processes. Both methods require precise control of parameters like temperature, concentration, and pH to minimize defects and optimize recovery efficiency.
Future Trends in Crystallization and Precipitation Technologies
Future trends in crystallization and precipitation technologies emphasize the integration of advanced process analytical technologies (PAT) and machine learning algorithms to optimize crystal size distribution and improve purity. The development of continuous crystallization and microfluidic precipitation systems supports enhanced control over nucleation and growth kinetics, leading to higher product consistency and scalability. Innovations in sustainable solvent selection and energy-efficient cooling methods further aim to reduce environmental impact and operational costs in industrial crystallization and precipitation processes.
Supersaturation
Supersaturation drives both crystallization and precipitation processes, where crystallization involves the orderly growth of solute molecules into a structured lattice from a supersaturated solution, while precipitation results in the rapid formation of solid particles without a defined crystal structure.
Nucleation
Nucleation in crystallization involves the formation of an organized crystal lattice from a supersaturated solution, whereas precipitation nucleation produces disordered solid particles without a defined crystal structure.
Solubility product (Ksp)
Crystallization occurs when a solution becomes saturated and solute molecules arrange into a solid lattice, while precipitation happens once the ionic product exceeds the solubility product constant (Ksp), causing an insoluble solid to form.
Metastable zone
The metastable zone defines the concentration and temperature range where crystallization occurs without spontaneous precipitation, allowing controlled crystal growth.
Primary nucleation
Primary nucleation in crystallization involves the spontaneous formation of a stable nucleus from a supersaturated solution, whereas precipitation primarily results from rapid solute aggregation without the ordered molecular arrangement characteristic of crystal formation.
Polymorphism
Crystallization and precipitation differ in polymorphism control, with crystallization enabling selective formation of specific polymorphs through controlled nucleation and growth conditions, while precipitation often results in a mixture of polymorphic forms due to rapid and less controlled solid formation.
Ostwald ripening
Ostwald ripening accelerates crystal growth during crystallization by dissolving smaller particles and redepositing material onto larger ones, unlike precipitation which forms solid particles abruptly from a supersaturated solution.
Seed crystals
Seed crystals initiate and guide crystallization by providing a structural template for orderly crystal growth, whereas precipitation often forms without such defined nucleation sites.
Solution-mediated transformation
Solution-mediated transformation occurs when a metastable phase dissolves and subsequently crystallizes into a more stable phase, distinguishing crystallization as a controlled, ordered process from precipitation, which often involves rapid, less ordered particle formation.
Induction time
Induction time in crystallization typically involves a measurable delay before nucleus formation under supersaturation, whereas precipitation often occurs rapidly with minimal induction time due to immediate nucleation.
Crystallization vs Precipitation Infographic
 njnir.com
njnir.com