Reverse osmosis employs a semi-permeable membrane to remove nearly all dissolved salts and impurities, achieving high levels of water purification. Nanofiltration uses membranes with larger pore sizes, selectively filtering out divalent ions and larger organic molecules while allowing monovalent salts to pass, making it ideal for softening and partial demineralization. The choice between reverse osmosis and nanofiltration depends on the required purity level, energy consumption, and specific separation needs in chemical engineering processes.
Table of Comparison
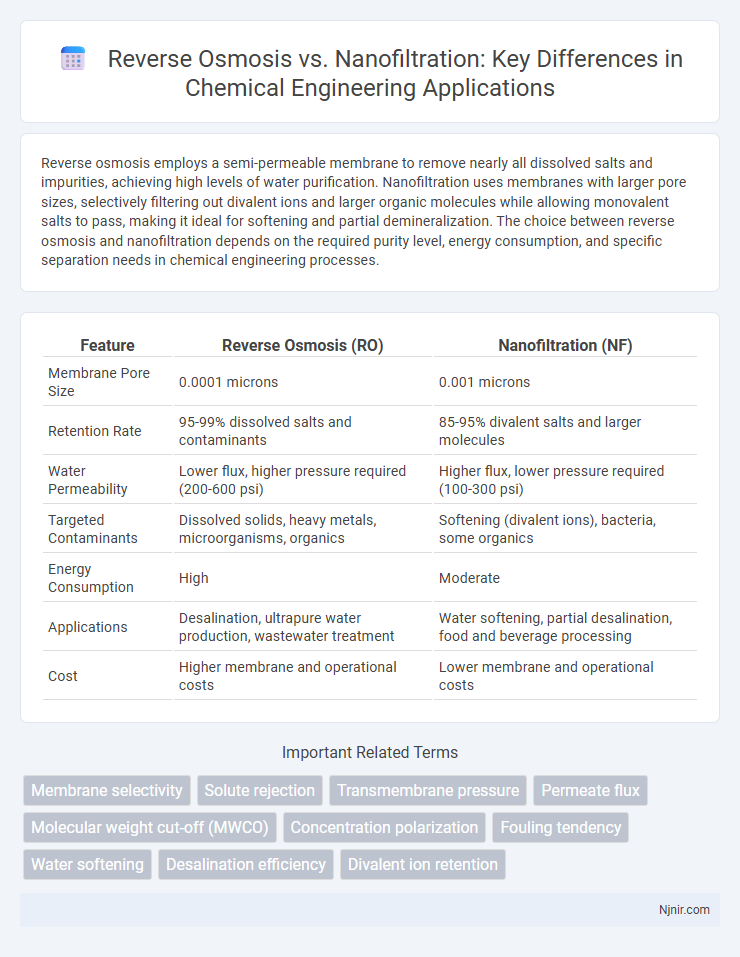
| Feature | Reverse Osmosis (RO) | Nanofiltration (NF) |
|---|---|---|
| Membrane Pore Size | 0.0001 microns | 0.001 microns |
| Retention Rate | 95-99% dissolved salts and contaminants | 85-95% divalent salts and larger molecules |
| Water Permeability | Lower flux, higher pressure required (200-600 psi) | Higher flux, lower pressure required (100-300 psi) |
| Targeted Contaminants | Dissolved solids, heavy metals, microorganisms, organics | Softening (divalent ions), bacteria, some organics |
| Energy Consumption | High | Moderate |
| Applications | Desalination, ultrapure water production, wastewater treatment | Water softening, partial desalination, food and beverage processing |
| Cost | Higher membrane and operational costs | Lower membrane and operational costs |
Introduction to Membrane Filtration Technologies
Reverse osmosis (RO) and nanofiltration (NF) are advanced membrane filtration technologies used for purifying water by removing contaminants and particles. RO membranes have smaller pore sizes, typically around 0.0001 microns, enabling them to filter out dissolved salts, viruses, and organic molecules, making them ideal for desalination and producing ultrapure water. Nanofiltration membranes feature slightly larger pores, approximately 0.001 microns, providing selective removal of divalent ions and larger organic molecules while allowing monovalent ions to pass, commonly used in softening water and removing specific contaminants without full desalination.
Fundamental Principles of Reverse Osmosis
Reverse osmosis operates on the principle of applying pressure to force water through a semipermeable membrane that blocks contaminants, salts, and larger molecules, allowing only pure water to pass. This process relies on the osmotic pressure difference to reverse the natural flow of water, effectively removing up to 99% of dissolved salts, organic molecules, and pathogens. The membrane's pore size is typically around 0.0001 microns, enabling highly selective filtration compared to nanofiltration, which has larger pores and primarily removes divalent ions and some organic compounds.
Core Mechanisms of Nanofiltration
Nanofiltration operates by using a semi-permeable membrane that selectively allows passage of monovalent ions and small molecules while rejecting divalent and larger ions based on size and charge exclusion. Its core mechanism involves both size sieving and electrostatic repulsion, effectively removing hardness ions like calcium and magnesium but allowing partial permeability to salts like sodium chloride. This contrasts with reverse osmosis, which relies primarily on pressure-driven diffusion through a tighter membrane to remove nearly all dissolved solutes and contaminants.
Comparison of Membrane Structures
Reverse osmosis (RO) membranes feature a dense, non-porous structure that blocks nearly all dissolved solids, including monovalent ions, providing high rejection rates typically above 99%. Nanofiltration (NF) membranes have a looser, selectively porous structure allowing passage of monovalent ions like sodium and chloride while rejecting divalent and larger ions, offering moderate rejection rates between 85% to 98%. The structural differences impact permeability, with NF membranes generally allowing higher flux and lower energy consumption compared to RO membranes due to their less restrictive architecture.
Selectivity and Rejection Rates
Reverse osmosis (RO) offers higher rejection rates, typically removing up to 99% of dissolved salts, organic compounds, and contaminants, making it ideal for producing ultrapure water. Nanofiltration (NF) exhibits selective permeability, efficiently rejecting divalent and larger molecules such as hardness ions and certain organic substances, while allowing monovalent ions like sodium and chloride to pass. The distinct selectivity and rejection profiles of RO and NF determine their suitability for applications ranging from desalination to water softening and specific contaminant removal.
Operating Pressure Requirements
Reverse osmosis membranes typically require higher operating pressures ranging from 150 to 600 psi to effectively remove dissolved salts and contaminants. Nanofiltration operates at a lower pressure range, usually between 70 to 150 psi, targeting divalent ions and larger molecules while allowing monovalent ions to pass through. The reduced pressure requirement of nanofiltration often results in lower energy consumption compared to reverse osmosis systems.
Energy Consumption and Efficiency
Reverse osmosis (RO) typically consumes more energy than nanofiltration (NF) due to its higher operating pressure requirements, often ranging between 8-16 bar compared to NF's 4-8 bar. RO achieves finer filtration by removing up to 99% of dissolved salts and contaminants, while NF targets larger molecules and divalent ions, offering higher permeability and lower fouling rates. Energy efficiency in NF systems improves overall water recovery rates by 10-20% and reduces operational costs, making NF preferable for applications where moderate solute rejection is acceptable.
Applications in Chemical Engineering Processes
Reverse osmosis (RO) is widely utilized in chemical engineering for high-purity water production, removing up to 99% of dissolved salts, organic compounds, and microorganisms, making it essential in pharmaceutical manufacturing and boiler feedwater treatment. Nanofiltration (NF) offers selective separation, effectively rejecting divalent and larger ions while allowing monovalent ions to pass, suitable for softening water, partial desalination, and separating organic molecules in chemical processing. Both technologies enhance process efficiency and product quality by targeting specific contaminants critical to chemical reactors, formulation processes, and wastewater reuse systems.
Advantages and Limitations of Each Technology
Reverse osmosis (RO) offers superior contaminant removal, effectively eliminating up to 99% of dissolved salts, heavy metals, and organic compounds, making it ideal for desalination and producing high-purity water; however, RO requires higher energy input and generates significant brine waste. Nanofiltration (NF) selectively removes divalent and larger molecules such as hardness ions and some organic compounds while preserving beneficial minerals, offering lower energy consumption and reduced membrane fouling compared to RO; its limitation lies in lower rejection rates for monovalent salts and pathogens. Both technologies play crucial roles in water treatment, with RO best suited for stringent purification and NF preferred for applications emphasizing energy efficiency and partial contaminant removal.
Recent Advances and Future Perspectives
Recent advances in reverse osmosis (RO) and nanofiltration (NF) technologies emphasize enhanced membrane materials like graphene oxide and thin-film composites, improving water permeability and salt rejection efficiency. Integration of energy-efficient methods such as pressure retarded osmosis and renewable energy sources is transforming desalination and wastewater treatment processes. Future perspectives highlight the development of smart membranes with self-cleaning capabilities and selective ion transport, aiming to reduce fouling and operational costs while expanding applications in pharmaceuticals and food industries.
Membrane selectivity
Reverse osmosis membranes exhibit higher selectivity by rejecting nearly all dissolved salts and contaminants, whereas nanofiltration membranes selectively remove divalent and larger molecules while allowing monovalent ions to pass through.
Solute rejection
Reverse osmosis achieves higher solute rejection rates, typically exceeding 99% for salts and small molecules, while nanofiltration selectively rejects divalent and larger solutes with rejection rates ranging from 85% to 99%, allowing monovalent ions to pass.
Transmembrane pressure
Reverse osmosis requires higher transmembrane pressure, typically 8-10 bar, compared to nanofiltration which operates efficiently at lower pressures around 4-6 bar.
Permeate flux
Nanofiltration typically achieves higher permeate flux rates than reverse osmosis due to its larger pore size and lower operating pressure requirements.
Molecular weight cut-off (MWCO)
Reverse osmosis membranes typically have a molecular weight cut-off (MWCO) of less than 100 Daltons, effectively removing salts and small organic molecules, while nanofiltration membranes have a higher MWCO ranging from 200 to 1000 Daltons, allowing partial passage of monovalent ions and targeting larger organic molecules.
Concentration polarization
Reverse osmosis membranes experience higher concentration polarization due to smaller pore sizes restricting solute passage compared to nanofiltration membranes, which exhibit lower concentration polarization effects allowing improved flux and reduced fouling.
Fouling tendency
Nanofiltration exhibits a lower fouling tendency than reverse osmosis due to its larger pore size and selective membrane structure that reduces particulate and organic matter accumulation.
Water softening
Nanofiltration effectively softens water by selectively removing divalent ions like calcium and magnesium, while reverse osmosis provides more comprehensive purification by eliminating nearly all dissolved salts, including monovalent ions.
Desalination efficiency
Reverse osmosis achieves higher desalination efficiency by effectively removing up to 99% of dissolved salts, while nanofiltration typically removes 90-98% but excels in selectively filtering divalent ions and organic compounds.
Divalent ion retention
Reverse osmosis membranes retain over 95% of divalent ions such as calcium and magnesium, while nanofiltration membranes typically retain 80-90%, making reverse osmosis more effective for divalent ion removal.
Reverse osmosis vs Nanofiltration Infographic
 njnir.com
njnir.com