Electrochemical synthesis uses electrical energy to drive chemical reactions, enabling precise control over reaction conditions and high selectivity in producing desired compounds. Photochemical synthesis relies on light energy to initiate reactions, often allowing the activation of specific molecular pathways that are difficult to achieve through thermal methods. Both approaches offer sustainable alternatives to traditional synthesis, with electrochemical methods excelling in energy efficiency and photochemical methods providing unique activation mechanisms.
Table of Comparison
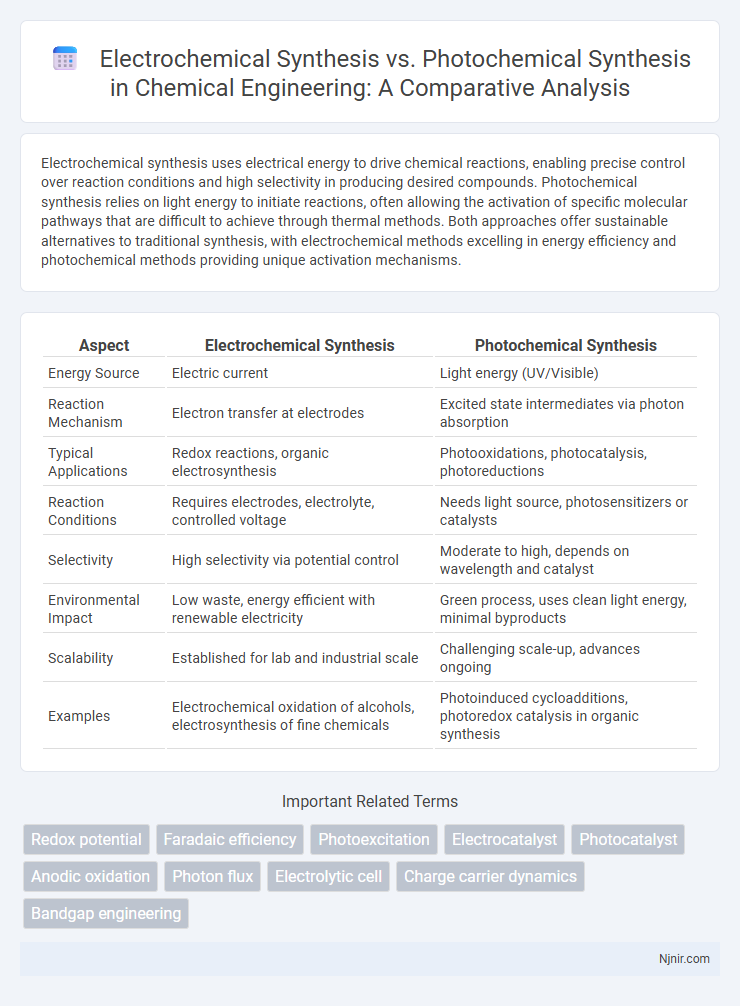
| Aspect | Electrochemical Synthesis | Photochemical Synthesis |
|---|---|---|
| Energy Source | Electric current | Light energy (UV/Visible) |
| Reaction Mechanism | Electron transfer at electrodes | Excited state intermediates via photon absorption |
| Typical Applications | Redox reactions, organic electrosynthesis | Photooxidations, photocatalysis, photoreductions |
| Reaction Conditions | Requires electrodes, electrolyte, controlled voltage | Needs light source, photosensitizers or catalysts |
| Selectivity | High selectivity via potential control | Moderate to high, depends on wavelength and catalyst |
| Environmental Impact | Low waste, energy efficient with renewable electricity | Green process, uses clean light energy, minimal byproducts |
| Scalability | Established for lab and industrial scale | Challenging scale-up, advances ongoing |
| Examples | Electrochemical oxidation of alcohols, electrosynthesis of fine chemicals | Photoinduced cycloadditions, photoredox catalysis in organic synthesis |
Introduction to Electrochemical and Photochemical Synthesis
Electrochemical synthesis harnesses electric current to drive chemical reactions, enabling precise control over redox processes and generating valuable organic and inorganic compounds efficiently. Photochemical synthesis employs light energy, typically from UV or visible sources, to activate molecules and induce chemical transformations that are often difficult to achieve thermally. Both methods offer sustainable and selective pathways for constructing complex molecules, with electrochemical synthesis emphasizing electron transfer mechanisms and photochemical synthesis relying on photon-induced excited states.
Fundamental Principles: Electrochemistry vs Photochemistry
Electrochemical synthesis relies on redox reactions driven by the flow of electrical current to induce chemical transformations, utilizing electrodes to facilitate electron transfer processes. Photochemical synthesis involves the absorption of photons to excite molecules to reactive states, initiating chemical changes through light-induced energy transfer or bond cleavage. Both methods harness non-thermal energy inputs, electrochemistry through applied voltage and photochemistry through specific wavelengths of light, enabling selective and efficient synthetic pathways.
Equipment and Setup Comparison
Electrochemical synthesis requires essential equipment including a power supply, electrodes (anode and cathode), electrochemical cells, and a reference electrode, necessitating precise control of voltage, current, and electrolyte composition for optimal reaction outcomes. Photochemical synthesis relies on light sources such as UV lamps or LEDs, reaction vessels transparent to the irradiation wavelength, and often requires cooling systems to manage heat generated during illumination. Compared to photochemical setups, electrochemical systems offer more straightforward scalability and integration with automation, while photochemical synthesis demands specialized optical components and careful design to ensure uniform light exposure and energy efficiency.
Reaction Mechanisms and Pathways
Electrochemical synthesis involves redox reactions driven by electron transfer at electrodes, enabling precise control of reaction pathways through modulation of applied potential and current density. Photochemical synthesis relies on light-induced excitation of molecules, generating reactive intermediates like radicals or excited states that follow distinct reaction mechanisms based on wavelength and photon energy. Both techniques enable activation of substrates under mild conditions but differ fundamentally in their initiation steps and energy input modes, influencing the selectivity and types of chemical transformations achievable.
Energy Sources: Electrical vs Light-Driven Processes
Electrochemical synthesis uses electrical energy to drive redox reactions through electron transfer at electrodes, enabling precise control over reaction conditions and energetics. Photochemical synthesis relies on light energy, typically from UV or visible sources, to excite molecules into reactive states that facilitate bond formation or cleavage. The choice between electrical and light-driven energy sources impacts reaction selectivity, scalability, and sustainability in organic and inorganic synthesis applications.
Applications in Organic and Inorganic Synthesis
Electrochemical synthesis enables precise redox control in organic and inorganic transformations, facilitating the formation of C-C bonds, oxidation of alcohols, and reduction of metal salts with high efficiency and sustainability. Photochemical synthesis harnesses light energy to initiate radical reactions, enabling selective functionalization of complex molecules, including cycloadditions and cross-couplings, often under mild conditions. Both methods offer greener alternatives to traditional syntheses, expanding the toolkit for pharmaceutical, polymer, and materials chemistry applications.
Environmental Impact and Sustainability
Electrochemical synthesis offers a greener alternative by using electricity--often sourced from renewable energy--to drive chemical reactions, significantly reducing hazardous waste and toxic by-products compared to traditional methods. Photochemical synthesis harnesses light energy, frequently solar, enabling energy-efficient processes that minimize reliance on fossil fuels and decrease carbon emissions. Both methodologies promote sustainable chemistry by enhancing reaction selectivity, reducing solvent usage, and enabling milder reaction conditions, thereby lowering environmental footprints.
Efficiency and Selectivity Analysis
Electrochemical synthesis offers high efficiency through direct electron transfer, enabling precise control over reaction parameters and minimizing energy waste, while photochemical synthesis harnesses light energy to drive reactions, often requiring specific wavelengths and photosensitizers that can limit overall efficiency. Electrochemical methods typically exhibit superior selectivity due to fine-tuned electrode potentials and reaction environments, which reduce side reactions and byproducts compared to photochemical processes that may produce reactive intermediates leading to lower selectivity. Both approaches provide green chemistry advantages, but electrochemical synthesis generally outperforms photochemical synthesis in terms of efficiency and selectivity for targeted organic transformations.
Challenges and Limitations
Electrochemical synthesis faces challenges including electrode material degradation, limited substrate scope, and difficulties in controlling selectivity due to competing redox processes. Photochemical synthesis encounters limitations such as low quantum efficiency, instability of photosensitizers, and dependence on light penetration, which restricts scalability and uniformity in reaction conditions. Both methods require careful optimization of reaction parameters to overcome these issues and achieve high yields and selectivity.
Future Perspectives and Emerging Trends
Electrochemical synthesis shows promise for scalable, sustainable chemical production with advances in electrode materials and flow cell technologies enhancing reaction efficiency and selectivity. Photochemical synthesis benefits from breakthroughs in photocatalyst design and solar-driven processes, enabling greener routes to complex molecules using visible light. Emerging trends highlight hybrid electro-photochemical systems combining both methods to optimize energy use and expand synthetic capabilities in pharmaceutical and materials science applications.
Redox potential
Electrochemical synthesis offers precise control over redox potential through applied voltage, enabling selective oxidation and reduction reactions, whereas photochemical synthesis relies on photon energy to generate reactive excited states with less direct tunability of redox potential.
Faradaic efficiency
Electrochemical synthesis generally achieves higher Faradaic efficiency than photochemical synthesis due to direct electron transfer minimizing energy loss during chemical transformations.
Photoexcitation
Photoexcitation in photochemical synthesis directly energizes molecular electrons to initiate reactions, whereas electrochemical synthesis relies on electron transfer via electrodes without photon involvement.
Electrocatalyst
Electrochemical synthesis leverages electrocatalysts to drive redox reactions efficiently at electrodes, enhancing selectivity and energy efficiency, while photochemical synthesis primarily relies on light-activated catalysts that promote electron transfer through photoexcitation.
Photocatalyst
Photochemical synthesis employs photocatalysts such as titanium dioxide or graphitic carbon nitride to efficiently harness light energy for driving chemical reactions under mild conditions.
Anodic oxidation
Anodic oxidation in electrochemical synthesis offers precise control over electron transfer and reaction conditions, enabling selective functionalization compared to photochemical synthesis, which relies on photon energy and often requires sensitizers for efficient oxidation.
Photon flux
Photon flux directly influences photochemical synthesis efficiency by controlling reaction rates, whereas electrochemical synthesis relies on electric current density rather than photon flux.
Electrolytic cell
Electrochemical synthesis using an electrolytic cell enables precise control over redox reactions by applying an external electric current to drive non-spontaneous chemical transformations efficiently.
Charge carrier dynamics
Electrochemical synthesis relies on controlled electron transfer at electrode interfaces influencing charge carrier mobility, while photochemical synthesis depends on photoexcited electron-hole pair generation and recombination dynamics affecting reaction efficiency.
Bandgap engineering
Electrochemical synthesis enables precise bandgap engineering by controlling redox potentials to tailor electronic structures, while photochemical synthesis uses light-induced excitation to modulate bandgap properties through photon energy absorption and electron-hole pair generation.
Electrochemical synthesis vs Photochemical synthesis Infographic
 njnir.com
njnir.com