Reactive distillation combines chemical reaction and separation in a single unit, enhancing efficiency by shifting reaction equilibrium and reducing equipment costs. Extractive distillation relies on a solvent to alter relative volatilities, improving the separation of azeotropic or close-boiling mixtures without chemical reactions. Choosing between reactive and extractive distillation depends on process requirements, feed composition, and economic considerations.
Table of Comparison
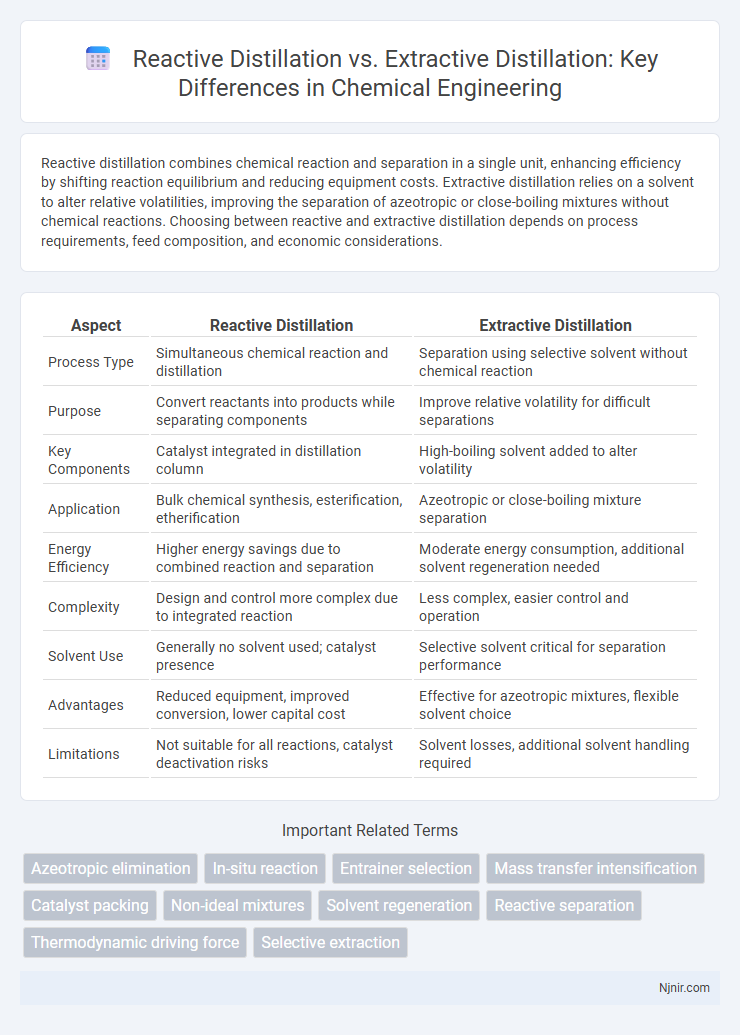
| Aspect | Reactive Distillation | Extractive Distillation |
|---|---|---|
| Process Type | Simultaneous chemical reaction and distillation | Separation using selective solvent without chemical reaction |
| Purpose | Convert reactants into products while separating components | Improve relative volatility for difficult separations |
| Key Components | Catalyst integrated in distillation column | High-boiling solvent added to alter volatility |
| Application | Bulk chemical synthesis, esterification, etherification | Azeotropic or close-boiling mixture separation |
| Energy Efficiency | Higher energy savings due to combined reaction and separation | Moderate energy consumption, additional solvent regeneration needed |
| Complexity | Design and control more complex due to integrated reaction | Less complex, easier control and operation |
| Solvent Use | Generally no solvent used; catalyst presence | Selective solvent critical for separation performance |
| Advantages | Reduced equipment, improved conversion, lower capital cost | Effective for azeotropic mixtures, flexible solvent choice |
| Limitations | Not suitable for all reactions, catalyst deactivation risks | Solvent losses, additional solvent handling required |
Introduction to Reactive and Extractive Distillation
Reactive distillation combines chemical reaction and separation processes within a single unit, enhancing efficiency by simultaneously converting and separating components, often used in esterification and etherification. Extractive distillation involves adding a solvent to alter relative volatilities of components, improving separation of azeotropic or close-boiling mixtures without changing their chemical nature. Both methods optimize process economics and energy consumption by integrating separation strategies tailored to specific mixture characteristics.
Fundamental Principles of Reactive Distillation
Reactive distillation integrates chemical reaction and separation into a single unit, enhancing efficiency by shifting reaction equilibrium through continuous removal of products. This process relies on catalysts within the distillation column, enabling simultaneous reaction and separation, which reduces energy consumption and equipment costs. The fundamental principle is leveraging reaction kinetics and volatility differences to achieve higher conversion and selectivity compared to conventional distillation methods.
Key Concepts in Extractive Distillation
Extractive distillation involves adding a high-boiling solvent to alter the relative volatility of components in a mixture, enhancing separation efficiency for close-boiling or azeotropic mixtures. The key concepts center on solvent selection, which must be non-volatile, selectively interact with one component, and remain largely unaltered during the process. The design also emphasizes solvent recovery, maintaining thermal stability, and optimizing the column configuration to achieve improved separation compared to conventional distillation methods.
Process Design Differences
Reactive distillation integrates chemical reaction and separation within a single column, combining reaction kinetics with distillation dynamics, leading to simplified process design and reduced equipment costs. Extractive distillation utilizes an additional solvent to alter relative volatilities, requiring careful solvent selection, solvent recovery systems, and more complex column internals to achieve effective separation. The key design difference lies in the simultaneous reaction-separation unit of reactive distillation versus the solvent-driven separation with separate reaction and distillation stages in extractive distillation.
Typical Applications in Industry
Reactive distillation is widely applied in processes requiring simultaneous chemical reaction and separation, such as esterification, etherification, and alkylation in the chemical and pharmaceutical industries. Extractive distillation is primarily used for separating azeotropes and close-boiling mixtures, common in petrochemical refining, solvent recovery, and the production of high-purity solvents. Industries leverage reactive distillation for enhanced conversion and energy efficiency, while extractive distillation is favored for precise component separation where traditional distillation fails.
Advantages of Reactive Distillation
Reactive distillation integrates chemical reaction and separation in a single unit, significantly reducing capital and operational costs by eliminating the need for separate reactors and distillation columns. This process enhances conversion and selectivity due to continuous removal of products, shifting equilibrium favorably and increasing overall efficiency. Reactive distillation also offers energy savings by utilizing heat generated from the reaction for separation, making it particularly advantageous in equilibrium-limited reactions.
Benefits of Extractive Distillation
Extractive distillation enhances separation of close-boiling or azeotropic mixtures by introducing a solvent that alters relative volatilities, leading to higher purity and yield compared to reactive distillation. It offers operational flexibility, allowing the recovery of valuable components without chemical reaction, thereby minimizing byproduct formation and simplifying downstream processing. Additionally, extractive distillation improves energy efficiency by reducing the number of theoretical stages needed for separation, resulting in lower operational costs.
Limitations and Technical Challenges
Reactive distillation faces limitations such as complex reaction-separation integration, catalyst deactivation, and difficulty controlling reaction kinetics alongside mass transfer, which complicates scale-up and process optimization. Extractive distillation encounters technical challenges including solvent selection for high selectivity and low volatility, managing solvent loss, and resolving corrosion and fouling issues in equipment due to aggressive solvents. Both processes require precise thermodynamic modeling and advanced control systems to address their operational complexities and improve industrial viability.
Energy and Cost Considerations
Reactive distillation integrates chemical reaction and separation in a single unit, significantly reducing energy consumption by eliminating the need for multiple heating and cooling stages, which also lowers capital and operating costs. Extractive distillation requires additional solvent recovery steps and typically involves higher energy input due to the need for separate distillation columns, resulting in increased operational expenses. Energy efficiency in reactive distillation often translates to cost savings, whereas extractive distillation's complexity and energy demand lead to higher overall expenditures.
Selection Criteria and Industrial Case Studies
Selection criteria for reactive distillation emphasize the integration of reaction and separation in a single unit, optimizing catalyst compatibility with distillation conditions and enabling energy-efficient processes for equilibrium-limited reactions like esterification and etherification. Extractive distillation requires careful solvent selection to alter relative volatilities, focusing on solvent volatility, selectivity, recovery ease, and thermal stability, commonly applied in separating close-boiling or azeotropic mixtures such as separating benzene-cyclohexane or ethanol-water. Industrial case studies highlight reactive distillation in methyl acetate production by combining esterification and separation, demonstrating reduced capital and operating costs, whereas extractive distillation is exemplified by the industrial drying of ethanol using ethylene glycol as a solvent, ensuring high purity product recovery.
Azeotropic elimination
Reactive distillation combines chemical reaction and separation in a single unit to break azeotropes efficiently, while extractive distillation uses a high-boiling solvent to alter relative volatilities and separate azeotropic mixtures without chemical reaction.
In-situ reaction
Reactive distillation combines chemical reaction and separation in a single column enabling in-situ reaction and enhanced conversion, whereas extractive distillation relies on adding a solvent for selective separation without in-situ chemical transformation.
Entrainer selection
Entrainer selection in extractive distillation prioritizes high selectivity and solvent recovery to enhance component separation, while reactive distillation requires catalysts that facilitate simultaneous reaction and separation without deactivating under process conditions.
Mass transfer intensification
Reactive distillation intensifies mass transfer by integrating chemical reactions with vapor-liquid separation in a single unit, whereas extractive distillation enhances mass transfer primarily through selective solvent addition to alter relative volatilities without reaction.
Catalyst packing
Catalyst packing in reactive distillation integrates reaction and separation within a single column, enhancing efficiency by simultaneously facilitating catalytic reactions and distillation, whereas extractive distillation uses non-catalytic packing combined with a solvent to alter relative volatilities for separation without chemical transformation.
Non-ideal mixtures
Reactive distillation integrates chemical reaction and separation in one unit operating efficiently with non-ideal mixtures by shifting equilibrium, whereas extractive distillation uses a high-boiling solvent to alter relative volatilities for enhanced separation of non-ideal mixtures without chemical reaction.
Solvent regeneration
Reactive distillation integrates chemical reaction and solvent regeneration within the same unit, enhancing efficiency, while extractive distillation typically requires separate solvent regeneration units, increasing operational complexity.
Reactive separation
Reactive distillation integrates chemical reaction and separation in a single unit, enhancing efficiency and selectivity by driving equilibrium-limited reactions to completion, whereas extractive distillation relies on adding solvents to alter relative volatilities for component separation without chemical transformation.
Thermodynamic driving force
Reactive distillation leverages simultaneous chemical reaction and separation to enhance the thermodynamic driving force by shifting equilibrium, while extractive distillation relies on adding a solvent to alter relative volatilities and increase the thermodynamic driving force for component separation.
Selective extraction
Reactive distillation integrates reaction and separation to enhance selective extraction by converting components in situ, while extractive distillation relies on selective solvents to separate components based on differences in volatility.
Reactive distillation vs Extractive distillation Infographic
 njnir.com
njnir.com