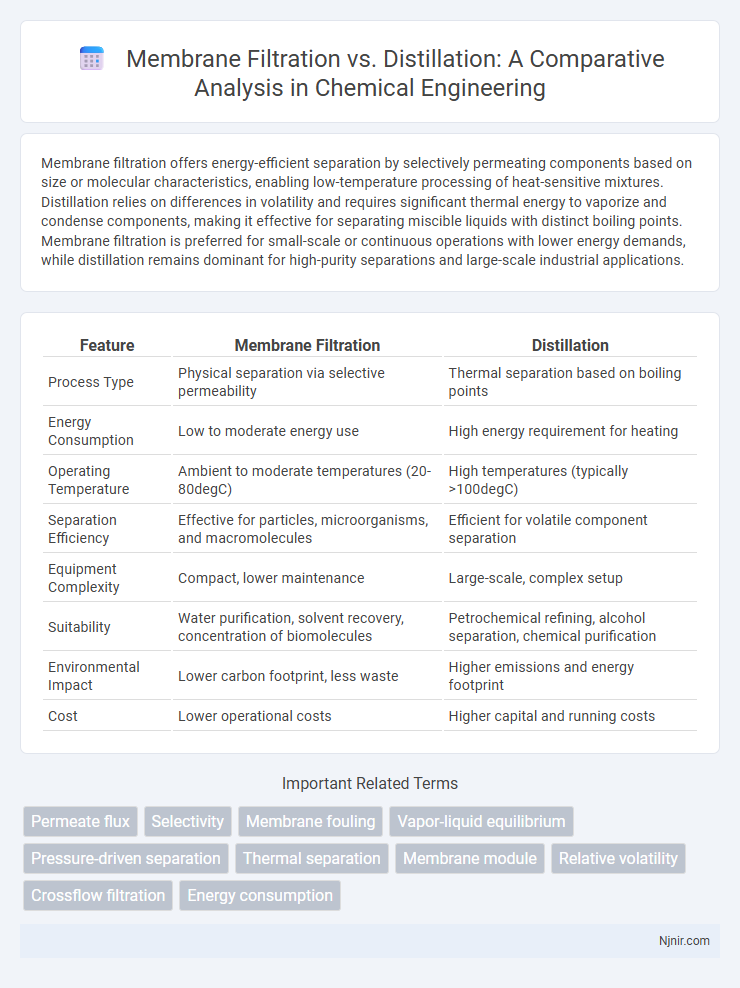
Membrane filtration offers energy-efficient separation by selectively permeating components based on size or molecular characteristics, enabling low-temperature processing of heat-sensitive mixtures. Distillation relies on differences in volatility and requires significant thermal energy to vaporize and condense components, making it effective for separating miscible liquids with distinct boiling points. Membrane filtration is preferred for small-scale or continuous operations with lower energy demands, while distillation remains dominant for high-purity separations and large-scale industrial applications.
Table of Comparison
| Feature | Membrane Filtration | Distillation |
|---|---|---|
| Process Type | Physical separation via selective permeability | Thermal separation based on boiling points |
| Energy Consumption | Low to moderate energy use | High energy requirement for heating |
| Operating Temperature | Ambient to moderate temperatures (20-80degC) | High temperatures (typically >100degC) |
| Separation Efficiency | Effective for particles, microorganisms, and macromolecules | Efficient for volatile component separation |
| Equipment Complexity | Compact, lower maintenance | Large-scale, complex setup |
| Suitability | Water purification, solvent recovery, concentration of biomolecules | Petrochemical refining, alcohol separation, chemical purification |
| Environmental Impact | Lower carbon footprint, less waste | Higher emissions and energy footprint |
| Cost | Lower operational costs | Higher capital and running costs |
Introduction to Membrane Filtration and Distillation
Membrane filtration uses semipermeable membranes to separate particles based on size and molecular properties, making it effective for water purification, wastewater treatment, and food processing. Distillation relies on vaporizing a liquid mixture and condensing its components based on differences in boiling points, commonly applied in chemical production, fuel refining, and desalination. Both techniques are essential in separation technology, with membrane filtration excelling in low-energy processes and distillation favored for high-purity separations.
Core Principles of Membrane Filtration
Membrane filtration operates on the principle of selective permeability, using semipermeable membranes to separate particles based on size, charge, or molecular weight. This process relies on pressure or concentration gradients to drive the separation, allowing water and small molecules to pass while retaining larger contaminants such as bacteria, viruses, and suspended solids. Common membrane types include microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, each designed for specific filtration degrees and applications.
Fundamentals of Distillation Processes
Distillation is a thermal separation process relying on differences in component volatilities to separate mixtures by selective vaporization and condensation, governed by phase equilibrium principles such as Raoult's and Dalton's laws. Key parameters influencing distillation efficiency include relative volatility, reflux ratio, number of theoretical stages, and column design, which determine the purity and recovery of components. Unlike membrane filtration that uses physical barriers to separate based on size or chemical affinity, distillation exploits thermodynamic properties and mass transfer between vapor and liquid phases.
Key Applications in Chemical Engineering
Membrane filtration is widely applied in chemical engineering for separation processes involving liquids, such as wastewater treatment, solvent recovery, and purification of chemical products, due to its energy efficiency and ability to selectively remove particles and contaminants. Distillation excels in applications requiring the separation of liquid mixtures based on boiling points, including petrochemical refining, alcohol production, and separation of organic solvents, where high purity and component recovery are critical. Both technologies are integral to process optimization, with membrane filtration favored for heat-sensitive materials and distillation preferred for complex mixtures requiring precise component separation.
Energy Efficiency: Membrane Filtration vs Distillation
Membrane filtration typically consumes significantly less energy than distillation due to its low thermal input requirements, relying primarily on pressure-driven processes rather than heat. Distillation demands substantial energy for phase changes, often making it less efficient, especially for separating heat-sensitive or dilute solutions. Advances in membrane technology continue to improve energy efficiency, positioning filtration as a cost-effective alternative in many industrial separation applications.
Separation Selectivity and Product Purity
Membrane filtration offers high separation selectivity by utilizing selective permeability of membranes to target specific molecules, resulting in products with high purity and minimal contamination. Distillation relies on differences in boiling points but often has lower selectivity for close-boiling compounds, potentially leading to less pure products and requiring multiple stages for enhanced purity. Membrane filtration is energy-efficient and suitable for heat-sensitive materials, whereas distillation is more versatile but energy-intensive and may cause thermal degradation.
Operational Costs and Economic Considerations
Membrane filtration generally offers lower operational costs due to reduced energy consumption, as it operates at moderate temperatures and pressures compared to distillation, which requires substantial heat input for phase change. Capital expenses for membrane systems can be higher initially, but maintenance and energy savings often lead to better long-term economic efficiency, especially for low to moderate throughput applications. Distillation remains cost-effective for large-scale separations with high purity demands despite higher ongoing energy costs and more complex equipment maintenance.
Environmental Impact and Sustainability
Membrane filtration consumes significantly less energy compared to distillation, reducing greenhouse gas emissions and operational costs in water and chemical processing industries. Unlike distillation, which relies on high thermal input and often uses fossil fuels, membrane processes utilize physical separation without phase change, contributing to lower carbon footprints and enhanced sustainability. Waste production is minimized in membrane filtration due to selective permeability, whereas distillation generates more concentrated brine and chemical residues, posing challenges for environmentally responsible disposal.
Technological Advances and Industry Trends
Membrane filtration technologies have evolved with innovations such as nanofiltration and forward osmosis, offering energy-efficient alternatives to traditional distillation by reducing thermal requirements and scaling challenges. Distillation advancements include multi-effect distillation and membrane distillation hybrid systems that improve separation efficiency and lower energy consumption in industrial applications. Industry trends highlight increasing adoption of membrane-based processes in water treatment and pharmaceutical sectors due to their precision and sustainability, while distillation remains essential for high-purity separations in petrochemical and chemical manufacturing.
Choosing the Right Process: Decision Factors
Membrane filtration offers energy-efficient separation for heat-sensitive products, while distillation excels in purifying components with significant boiling point differences. Key decision factors include feed composition, desired purity, operational costs, and thermal stability of the mixture. Evaluating these parameters ensures optimal process selection for industrial applications.
Permeate flux
Membrane filtration achieves higher permeate flux rates than distillation by enabling selective separation at lower temperatures and pressures, resulting in greater energy efficiency and faster processing times.
Selectivity
Membrane filtration offers higher selectivity for separating specific molecules based on size and charge, while distillation relies on differences in boiling points to achieve separation.
Membrane fouling
Membrane filtration efficiency is significantly reduced by membrane fouling, which causes pore blockage and surface contamination, unlike distillation that primarily encounters energy-intensive phase change challenges.
Vapor-liquid equilibrium
Membrane filtration separates components based on size and permeability without altering vapor-liquid equilibrium, whereas distillation relies on differences in vapor-liquid equilibrium to separate mixtures through phase change and selective vaporization.
Pressure-driven separation
Membrane filtration utilizes pressure-driven separation by forcing fluid through selective membranes to remove contaminants, whereas distillation relies on thermal energy to vaporize and condense components based on boiling points.
Thermal separation
Membrane filtration achieves separation by selective permeability without phase change, whereas distillation relies on thermal separation through boiling and condensation to separate components based on volatility differences.
Membrane module
Membrane filtration modules offer energy-efficient separation by using selective semipermeable membranes to remove contaminants from liquids, providing advantages over distillation in preserving heat-sensitive compounds and reducing operational costs.
Relative volatility
Membrane filtration separates components based on size and permeability, whereas distillation relies on differences in relative volatility to separate mixtures through vapor-liquid equilibrium.
Crossflow filtration
Crossflow membrane filtration offers higher efficiency and lower energy consumption compared to distillation by continuously removing particles through tangential flow, reducing fouling and enhancing separation performance in liquid processing.
Energy consumption
Membrane filtration consumes significantly less energy than distillation by operating at lower temperatures and pressures, making it a more energy-efficient separation method for liquid mixtures.
Membrane filtration vs Distillation Infographic
 njnir.com
njnir.com