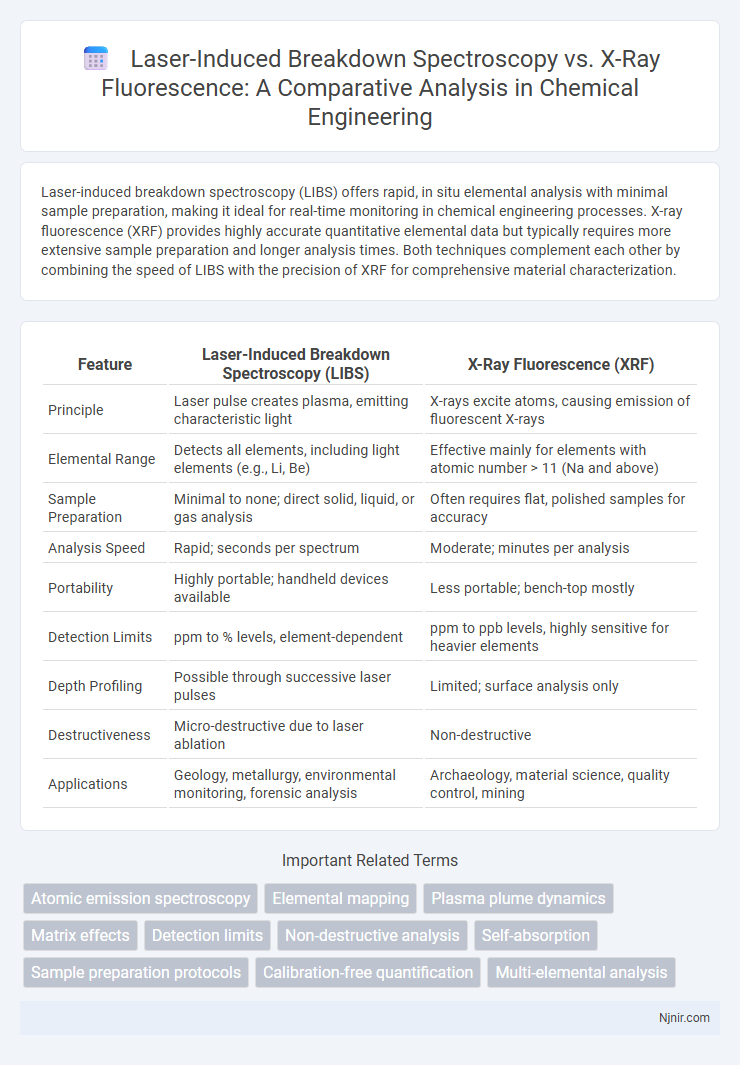
Laser-induced breakdown spectroscopy (LIBS) offers rapid, in situ elemental analysis with minimal sample preparation, making it ideal for real-time monitoring in chemical engineering processes. X-ray fluorescence (XRF) provides highly accurate quantitative elemental data but typically requires more extensive sample preparation and longer analysis times. Both techniques complement each other by combining the speed of LIBS with the precision of XRF for comprehensive material characterization.
Table of Comparison
| Feature | Laser-Induced Breakdown Spectroscopy (LIBS) | X-Ray Fluorescence (XRF) |
|---|---|---|
| Principle | Laser pulse creates plasma, emitting characteristic light | X-rays excite atoms, causing emission of fluorescent X-rays |
| Elemental Range | Detects all elements, including light elements (e.g., Li, Be) | Effective mainly for elements with atomic number > 11 (Na and above) |
| Sample Preparation | Minimal to none; direct solid, liquid, or gas analysis | Often requires flat, polished samples for accuracy |
| Analysis Speed | Rapid; seconds per spectrum | Moderate; minutes per analysis |
| Portability | Highly portable; handheld devices available | Less portable; bench-top mostly |
| Detection Limits | ppm to % levels, element-dependent | ppm to ppb levels, highly sensitive for heavier elements |
| Depth Profiling | Possible through successive laser pulses | Limited; surface analysis only |
| Destructiveness | Micro-destructive due to laser ablation | Non-destructive |
| Applications | Geology, metallurgy, environmental monitoring, forensic analysis | Archaeology, material science, quality control, mining |
Introduction to Analytical Techniques in Chemical Engineering
Laser-induced breakdown spectroscopy (LIBS) and X-ray fluorescence (XRF) are advanced analytical techniques widely used in chemical engineering for elemental analysis. LIBS employs a high-energy laser pulse to create a plasma on the sample surface, enabling rapid, in situ qualitative and quantitative measurements with minimal sample preparation. XRF utilizes X-ray excitation to induce characteristic secondary (fluorescent) X-rays from elements in the sample, offering precise, non-destructive analysis with high sensitivity for major and trace elements in complex matrices.
Overview of Laser-Induced Breakdown Spectroscopy (LIBS)
Laser-Induced Breakdown Spectroscopy (LIBS) utilizes a high-energy laser pulse to ablate a small amount of material, creating a plasma whose emitted light is analyzed to determine elemental composition. LIBS offers rapid, real-time, and minimally destructive analysis with the capability to detect light elements such as hydrogen, lithium, and boron that are challenging for X-ray fluorescence (XRF) to measure accurately. Compared to XRF, LIBS provides enhanced surface sensitivity and in situ analysis without the need for vacuum conditions, making it suitable for diverse applications ranging from environmental monitoring to industrial quality control.
Fundamentals of X-ray Fluorescence (XRF)
X-ray fluorescence (XRF) operates on the principle of stimulating inner-shell electrons in atoms by irradiating a sample with primary X-rays, causing the emission of secondary X-rays characteristic of specific elements. The energy and intensity of these emitted X-rays provide qualitative and quantitative elemental analysis with high precision and minimal sample preparation. XRF is widely used in material science, geochemistry, and metallurgy for non-destructive elemental identification and composition analysis.
Sample Preparation Requirements: LIBS vs XRF
Laser-induced breakdown spectroscopy (LIBS) requires minimal sample preparation, allowing direct analysis of solids, liquids, and gases without extensive preprocessing. X-ray fluorescence (XRF) often demands more thorough sample preparation, including grinding, pelletizing, or pressing powders to ensure homogeneity and accurate results. LIBS advantages include rapid, in situ measurements, whereas XRF's preparation-intensive process contributes to higher accuracy for elemental composition in lab settings.
Elemental Detection Capabilities and Sensitivity
Laser-induced breakdown spectroscopy (LIBS) offers rapid, in-situ elemental detection with high spatial resolution, capable of analyzing light elements such as hydrogen, lithium, and beryllium, which X-ray fluorescence (XRF) struggles to detect effectively. XRF provides highly sensitive and accurate quantitative analysis for heavier elements, especially metals, with minimal sample preparation but is limited in detecting elements with low atomic numbers. The detection limits of LIBS typically range from parts per million (ppm) to parts per billion (ppb), while XRF achieves lower detection limits for heavy elements but lacks sensitivity for light elements, making both techniques complementary depending on the targeted elemental composition.
Quantitative and Qualitative Analysis Comparison
Laser-induced breakdown spectroscopy (LIBS) offers rapid elemental analysis with minimal sample preparation and is effective for detecting light elements such as hydrogen, carbon, and oxygen, providing both qualitative and quantitative data. X-ray fluorescence (XRF) excels in quantitative analysis of heavier elements with high accuracy and precision, particularly useful in bulk material characterization but struggles with light elements and requires flat, homogeneous samples. LIBS enables micro-scale spatial analysis and depth profiling, whereas XRF is limited to surface composition, making LIBS more versatile for in-situ and heterogeneous sample investigations.
Applications in Chemical Engineering Processes
Laser-induced breakdown spectroscopy (LIBS) enables real-time, in situ elemental analysis in chemical engineering, providing rapid monitoring of process variables such as catalyst composition and corrosion detection. X-ray fluorescence (XRF) offers precise bulk composition analysis crucial for quality control in material synthesis and alloy production. Combining LIBS for surface-level analysis with XRF for detailed bulk characterization enhances process optimization and ensures compliance with stringent chemical engineering standards.
Advantages and Limitations of LIBS and XRF
Laser-induced breakdown spectroscopy (LIBS) offers rapid, in-situ elemental analysis with minimal sample preparation and the ability to detect light elements like hydrogen and lithium, which X-ray fluorescence (XRF) often misses. LIBS limitations include its relatively higher detection limits, matrix effects, and potential sample damage due to laser ablation, whereas XRF provides excellent sensitivity for heavy elements and non-destructive analysis but struggles with light element detection and requires more complex sample prep for heterogeneous samples. Both techniques complement each other in varied applications, with LIBS excelling in portability and real-time analysis and XRF in high-precision quantitative elemental determination.
Safety, Cost, and Operational Considerations
Laser-induced breakdown spectroscopy (LIBS) offers enhanced safety due to its remote sensing capability and minimal sample preparation, reducing operator exposure to harmful substances, whereas X-ray fluorescence (XRF) involves direct exposure to X-ray radiation, necessitating strict safety protocols. From a cost perspective, LIBS systems tend to have lower initial investment and maintenance expenses compared to XRF, which requires more expensive detectors and shielding housing. Operationally, LIBS provides rapid, real-time analysis with minimal sample damage, making it suitable for diverse environments, while XRF typically offers higher elemental sensitivity but requires stable power sources and controlled environments.
Future Trends and Emerging Innovations
Laser-induced breakdown spectroscopy (LIBS) continues to advance with innovations like ultrafast lasers and enhanced data analytics, enabling rapid, in-situ elemental analysis with minimal sample preparation. Emerging trends in X-ray fluorescence (XRF) include the integration of portable devices and AI-driven spectral interpretation, improving field applicability and accuracy for complex matrices. Both technologies are converging towards hybrid systems combining LIBS's molecular analysis and XRF's precise elemental quantification to meet growing demands in environmental and industrial monitoring.
Atomic emission spectroscopy
Laser-induced breakdown spectroscopy (LIBS) provides rapid, in situ atomic emission spectroscopy by generating plasma to analyze elemental composition, whereas X-ray fluorescence (XRF) relies on secondary X-ray emission and is less effective for light element detection.
Elemental mapping
Laser-induced breakdown spectroscopy enables rapid, high-resolution elemental mapping with minimal sample preparation, while X-ray fluorescence offers precise, non-destructive elemental analysis over larger areas but with lower spatial resolution.
Plasma plume dynamics
Laser-induced breakdown spectroscopy exhibits complex plasma plume dynamics characterized by rapid expansion and cooling, which influence elemental emission intensity differently compared to the relatively static excitation environment in X-ray fluorescence analysis.
Matrix effects
Laser-induced breakdown spectroscopy exhibits stronger matrix effects than X-ray fluorescence due to its reliance on plasma formation influenced by sample composition and physical properties.
Detection limits
Laser-induced breakdown spectroscopy offers detection limits typically in the parts-per-million range, whereas X-ray fluorescence achieves superior detection limits often reaching parts-per-billion, making XRF more sensitive for trace element analysis.
Non-destructive analysis
Laser-induced breakdown spectroscopy offers rapid, in situ non-destructive elemental analysis with minimal sample preparation, while X-ray fluorescence provides highly accurate, non-destructive bulk elemental composition suitable for diverse materials.
Self-absorption
Laser-induced breakdown spectroscopy often experiences self-absorption effects that reduce spectral line intensity and accuracy, whereas X-ray fluorescence generally exhibits minimal self-absorption, leading to more reliable elemental quantification.
Sample preparation protocols
Laser-induced breakdown spectroscopy requires minimal to no sample preparation, allowing rapid analysis of solid, liquid, and powdered samples, whereas X-ray fluorescence demands rigorous sample preparation including homogenization, pelletizing, or fusion to ensure uniformity and accuracy in elemental analysis.
Calibration-free quantification
Laser-induced breakdown spectroscopy enables calibration-free quantification by directly analyzing elemental emission spectra, while X-ray fluorescence relies on calibrated standards for accurate elemental analysis.
Multi-elemental analysis
Laser-induced breakdown spectroscopy offers rapid, in-situ multi-elemental analysis with minimal sample preparation, whereas X-ray fluorescence provides high sensitivity and precision for quantitative elemental composition but often requires more extensive sample handling.
laser-induced breakdown spectroscopy vs X-ray fluorescence Infographic
 njnir.com
njnir.com