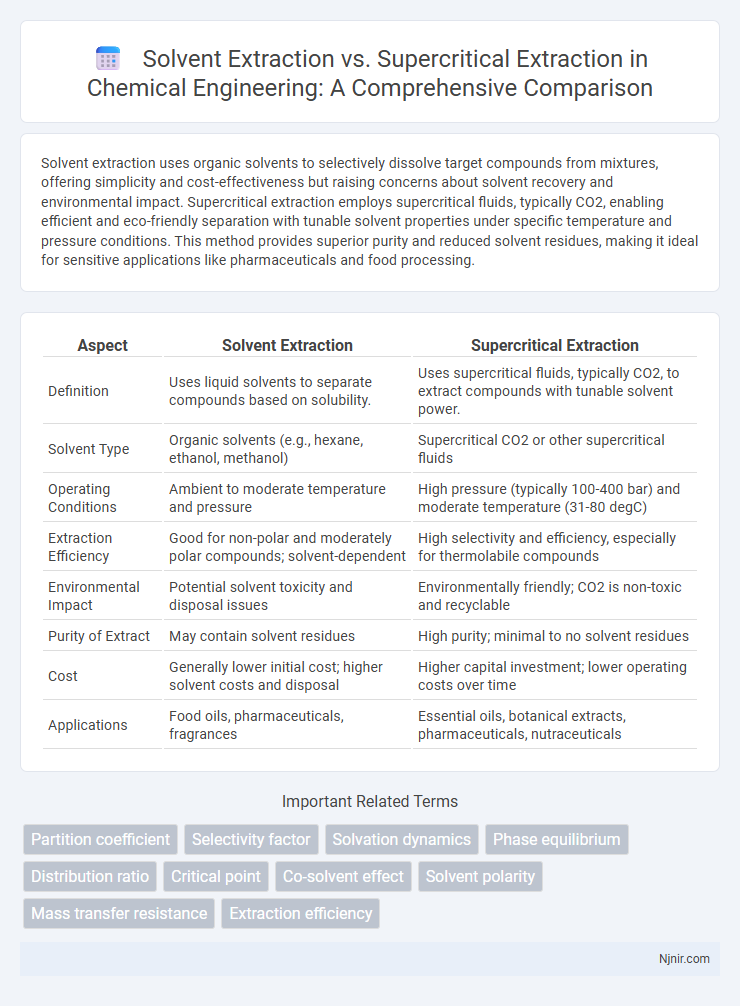
Solvent extraction uses organic solvents to selectively dissolve target compounds from mixtures, offering simplicity and cost-effectiveness but raising concerns about solvent recovery and environmental impact. Supercritical extraction employs supercritical fluids, typically CO2, enabling efficient and eco-friendly separation with tunable solvent properties under specific temperature and pressure conditions. This method provides superior purity and reduced solvent residues, making it ideal for sensitive applications like pharmaceuticals and food processing.
Table of Comparison
| Aspect | Solvent Extraction | Supercritical Extraction |
|---|---|---|
| Definition | Uses liquid solvents to separate compounds based on solubility. | Uses supercritical fluids, typically CO2, to extract compounds with tunable solvent power. |
| Solvent Type | Organic solvents (e.g., hexane, ethanol, methanol) | Supercritical CO2 or other supercritical fluids |
| Operating Conditions | Ambient to moderate temperature and pressure | High pressure (typically 100-400 bar) and moderate temperature (31-80 degC) |
| Extraction Efficiency | Good for non-polar and moderately polar compounds; solvent-dependent | High selectivity and efficiency, especially for thermolabile compounds |
| Environmental Impact | Potential solvent toxicity and disposal issues | Environmentally friendly; CO2 is non-toxic and recyclable |
| Purity of Extract | May contain solvent residues | High purity; minimal to no solvent residues |
| Cost | Generally lower initial cost; higher solvent costs and disposal | Higher capital investment; lower operating costs over time |
| Applications | Food oils, pharmaceuticals, fragrances | Essential oils, botanical extracts, pharmaceuticals, nutraceuticals |
Introduction to Solvent and Supercritical Extraction
Solvent extraction utilizes liquid solvents to selectively dissolve target compounds from raw materials, relying on differences in solubility and affinity to separate desired components efficiently. Supercritical extraction employs supercritical fluids, often supercritical CO2, combining gas-like diffusivity and liquid-like solvating power to extract bioactive compounds without thermal degradation. Both techniques optimize compound recovery, with solvent extraction favored for its simplicity and supercritical extraction valued for its environmental safety and selectivity.
Principles of Solvent Extraction
Solvent extraction operates on the principle of selective solubility, where a solvent dissolves specific components from a mixture based on their differing chemical affinity and polarity. This process relies on liquid-liquid phase distribution, allowing target compounds to migrate into the solvent phase for separation and recovery. In contrast, supercritical extraction utilizes supercritical fluids, often carbon dioxide, exploiting their tunable solvating power near critical temperature and pressure conditions for selective extraction without residual solvents.
Fundamentals of Supercritical Fluid Extraction
Supercritical fluid extraction (SFE) utilizes supercritical fluids, typically CO2 above its critical temperature and pressure, to selectively dissolve and extract target compounds with enhanced efficiency and minimal solvent residue compared to traditional solvent extraction. The tunable solvating power of supercritical CO2 allows precise control over extraction selectivity and yield, making SFE ideal for thermally sensitive or complex samples. Fundamental parameters including temperature, pressure, and co-solvent use critically influence solute solubility and mass transfer rates in supercritical fluid extraction processes.
Common Solvents and Supercritical Fluids Used
Solvent extraction commonly employs organic solvents such as hexane, ethanol, and methanol to dissolve desired compounds, leveraging their polarity and solubility characteristics for efficient separation. Supercritical extraction primarily uses supercritical carbon dioxide (CO2) due to its tunable solvating power, low toxicity, and environmentally friendly profile, occasionally enhanced with co-solvents like ethanol to improve solubility of polar compounds. The choice of solvent or supercritical fluid directly impacts extraction efficiency, selectivity, and environmental safety in applications ranging from pharmaceuticals to food processing.
Process Design and Equipment Comparison
Solvent extraction utilizes organic solvents like hexane to dissolve target compounds, requiring large extraction vessels, evaporators, and solvent recovery systems, while supercritical extraction employs supercritical CO2 under high pressure and temperature in specialized high-pressure vessels and separators. Process design for solvent extraction involves multiple stages of solvent contact, phase separation, and solvent recycling, often resulting in higher solvent consumption and potential environmental concerns. In contrast, supercritical extraction offers precise tunability of solvent properties for selective extraction, faster mass transfer rates, and cleaner product streams but demands more complex, costly high-pressure equipment and stringent safety measures.
Extraction Efficiency and Selectivity
Solvent extraction offers high extraction efficiency by dissolving a wide range of compounds but often lacks selectivity, leading to co-extraction of unwanted substances. Supercritical extraction, particularly with CO2, provides enhanced selectivity due to tunable solvent properties under varying temperature and pressure, resulting in purer extracts with minimal impurities. Efficiency in supercritical extraction depends on precise control of operational parameters, enabling targeted extraction of specific bioactive compounds while minimizing solvent residues.
Environmental and Safety Considerations
Solvent extraction involves the use of organic solvents, which pose risks of toxicity, flammability, and environmental pollution due to solvent residue and emissions. Supercritical extraction, typically using supercritical CO2, is safer and more environmentally friendly, as it is non-toxic, non-flammable, and leaves no harmful residues, significantly reducing waste and air contamination. The selection of supercritical extraction enhances operational safety and sustainability by minimizing hazardous chemical exposure and ecological impact.
Energy Consumption and Operational Costs
Solvent extraction typically involves higher energy consumption due to the need for extensive heating and solvent recovery processes, increasing operational costs significantly. Supercritical extraction, which uses supercritical fluids like CO2 at moderate temperatures and pressures, offers lower energy requirements and reduces solvent usage, leading to decreased operational expenses. The efficiency of supercritical extraction in minimizing waste and simplifying solvent recycling results in cost-effectiveness despite higher initial equipment investments.
Industrial Applications and Case Studies
Solvent extraction in industrial applications is widely used for oil recovery from seeds and pharmaceuticals, offering cost-effective processing with extensive scalability, as seen in soybean oil and essential oil industries. Supercritical extraction, particularly supercritical CO2 extraction, demonstrates superior selectivity and purity in extracting bioactive compounds for food, cosmetics, and pharmaceutical sectors, exemplified by its use in decaffeinating coffee and producing high-purity botanical extracts. Case studies reveal solvent extraction excels in bulk commodity processing while supercritical extraction leads in high-value, sensitive compound isolation, emphasizing the choice depends on product specificity, environmental impact, and operational costs.
Future Prospects and Technological Advancements
Supercritical extraction leverages CO2 under critical conditions, offering enhanced selectivity and environmental benefits compared to traditional solvent extraction, which relies on organic solvents with potential toxicity and residue issues. Future prospects emphasize integrating green solvents and hybrid techniques to increase efficiency and reduce environmental impact in solvent extraction, while advancements in supercritical extraction focus on process intensification through improved pressure and temperature controls, as well as scalable continuous-flow systems. Emerging technologies such as real-time monitoring using spectroscopy and AI-driven process optimization are poised to revolutionize both extraction methods, driving higher yield, purity, and sustainability in pharmaceutical, nutraceutical, and flavor industries.
Partition coefficient
Solvent extraction relies on the partition coefficient to quantify solute distribution between phases, whereas supercritical extraction exploits tunable solvent density and diffusivity, enhancing solute partitioning efficiency beyond conventional solvent limitations.
Selectivity factor
Supercritical extraction offers a higher selectivity factor than solvent extraction by precisely tuning temperature and pressure to target specific compounds without co-extracting impurities.
Solvation dynamics
Solvent extraction relies on solvation dynamics driven by liquid-phase interactions, while supercritical extraction exploits tunable solvent density and diffusivity in supercritical fluids to enhance solvation and mass transfer rates.
Phase equilibrium
Supercritical extraction offers precise phase equilibrium control due to tunable solvent density and solubility parameters, whereas solvent extraction relies on fixed liquid-liquid phase equilibria often limiting selectivity and efficiency.
Distribution ratio
The distribution ratio in solvent extraction significantly depends on the solute's partition between two immiscible liquids, while in supercritical extraction it varies based on solute solubility and density differences in supercritical fluids.
Critical point
Solvent extraction operates at ambient conditions without reaching a critical point, whereas supercritical extraction utilizes temperatures and pressures above a substance's critical point to achieve enhanced solvent properties.
Co-solvent effect
Co-solvent in supercritical extraction enhances solubility and selectivity of target compounds compared to traditional solvent extraction by modifying solvent polarity and improving mass transfer efficiency.
Solvent polarity
Solvent extraction efficiency varies significantly with solvent polarity, as polar solvents extract polar compounds effectively, whereas supercritical extraction uses nonpolar supercritical CO2, favoring nonpolar substance extraction.
Mass transfer resistance
Supercritical extraction exhibits lower mass transfer resistance than solvent extraction due to enhanced diffusivity and reduced viscosity of supercritical fluids, enabling faster solute transport and improved extraction efficiency.
Extraction efficiency
Supercritical extraction achieves higher extraction efficiency than solvent extraction by utilizing tunable solvent properties under supercritical conditions, allowing for selective and rapid solute recovery with minimal solvent residues.
Solvent extraction vs Supercritical extraction Infographic
 njnir.com
njnir.com