Green chemistry prioritizes the use of sustainable, non-toxic materials and energy-efficient processes to minimize environmental impact and reduce hazardous waste generation. Conventional synthesis often relies on toxic solvents, heavy metals, and energy-intensive conditions that contribute to pollution and resource depletion. Implementing green chemistry principles in chemical engineering enhances process safety, reduces costs, and promotes circular economy practices by utilizing renewable feedstocks and designing degradable products.
Table of Comparison
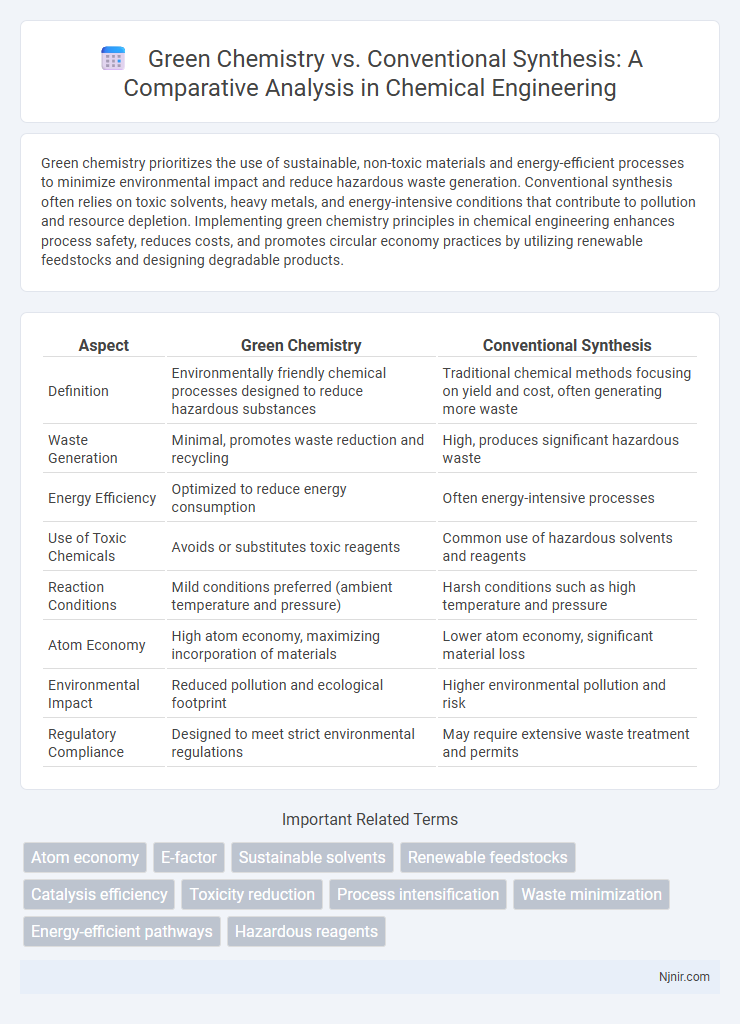
| Aspect | Green Chemistry | Conventional Synthesis |
|---|---|---|
| Definition | Environmentally friendly chemical processes designed to reduce hazardous substances | Traditional chemical methods focusing on yield and cost, often generating more waste |
| Waste Generation | Minimal, promotes waste reduction and recycling | High, produces significant hazardous waste |
| Energy Efficiency | Optimized to reduce energy consumption | Often energy-intensive processes |
| Use of Toxic Chemicals | Avoids or substitutes toxic reagents | Common use of hazardous solvents and reagents |
| Reaction Conditions | Mild conditions preferred (ambient temperature and pressure) | Harsh conditions such as high temperature and pressure |
| Atom Economy | High atom economy, maximizing incorporation of materials | Lower atom economy, significant material loss |
| Environmental Impact | Reduced pollution and ecological footprint | Higher environmental pollution and risk |
| Regulatory Compliance | Designed to meet strict environmental regulations | May require extensive waste treatment and permits |
Introduction to Green Chemistry and Conventional Synthesis
Green chemistry emphasizes the design of chemical processes that reduce or eliminate hazardous substances, focusing on sustainability, resource efficiency, and environmental impact reduction. Conventional synthesis often involves multi-step reactions with toxic reagents, high energy consumption, and generation of hazardous waste. Green chemistry principles prioritize renewable feedstocks, safer solvents, and energy-efficient methods to minimize ecological footprints and enhance process safety.
Core Principles of Green Chemistry
Green chemistry emphasizes waste prevention, use of safer solvents, and energy-efficient processes, contrasting with conventional synthesis that often relies on hazardous reagents and generates significant waste. Core principles include atom economy, using renewable feedstocks, and designing for degradation, which minimize environmental impact and enhance sustainability. These principles drive innovation in chemical manufacturing by prioritizing safety, resource efficiency, and reduced toxicity.
Key Methods in Conventional Chemical Synthesis
Conventional chemical synthesis typically relies on stoichiometric reagents and harsh reaction conditions, such as strong acids, bases, or heavy metal catalysts, which often generate toxic byproducts and significant waste. Key methods include classical organic reactions like Friedel-Crafts acylation, Grignard reactions, and chlorination, which prioritize yield and reactivity but frequently compromise environmental safety. These traditional approaches contrast with green chemistry techniques that emphasize atom economy, renewable feedstocks, and benign solvents to minimize ecological impact.
Environmental Impact: Green vs Conventional Approaches
Green chemistry techniques significantly reduce hazardous waste and lower the carbon footprint compared to conventional synthesis, which often relies on toxic solvents and generates large amounts of non-biodegradable byproducts. The use of renewable feedstocks and energy-efficient catalytic processes in green chemistry minimizes environmental pollution and conserves natural resources. Conventional synthesis methods typically involve energy-intensive steps and toxic reagent disposal, leading to severe environmental contamination and increased health risks.
Resource Efficiency and Waste Generation
Green chemistry prioritizes resource efficiency by using renewable feedstocks and designing processes that minimize energy consumption, significantly reducing raw material input compared to conventional synthesis. It generates substantially less hazardous waste through atom economy principles and safer reaction conditions, enhancing environmental sustainability. Conventional synthesis often relies on non-renewable resources and produces large quantities of toxic byproducts, leading to higher waste management costs and ecological impact.
Energy Consumption and Sustainability
Green chemistry significantly reduces energy consumption by employing catalysts, ambient reaction conditions, and renewable feedstocks, thus minimizing environmental impact and enhancing sustainability. Conventional synthesis often relies on high temperatures, pressures, and toxic solvents, leading to greater energy demands and increased waste generation. Sustainable practices in green chemistry align with global goals of reducing carbon footprints and promoting resource efficiency in chemical manufacturing.
Toxicity and Safety Considerations
Green chemistry prioritizes the use of non-toxic, renewable materials and safer solvents to minimize hazardous waste and exposure risks, significantly reducing environmental and human health hazards compared to conventional synthesis. Conventional synthesis often relies on toxic reagents, heavy metals, and volatile organic compounds that pose significant safety risks and generate hazardous byproducts requiring specialized disposal methods. Implementing green chemistry principles enhances process safety by lowering toxicity levels and minimizing the potential for accidents and long-term contamination in chemical manufacturing.
Economic Viability and Scalability
Green chemistry reduces production costs by minimizing waste and energy consumption, enhancing economic viability compared to conventional synthesis, which often involves expensive raw materials and hazardous reagents. Scalability in green chemistry benefits from simpler reaction conditions and safer processes, allowing easier transition from lab to industrial scale. Conventional synthesis faces challenges in scalability due to complex purification steps and environmental regulations increasing operational costs.
Case Studies: Green Chemistry in Industrial Applications
Case studies in industrial applications demonstrate that green chemistry significantly reduces hazardous waste and energy consumption compared to conventional synthesis. For example, Dow Chemical's bio-based 1,3-propanediol production uses renewable feedstocks and biocatalysis to replace petrochemical processes, enhancing sustainability and cost efficiency. Pfizer's adoption of catalytic enantioselective synthesis methods minimizes toxic solvents and improves atom economy, showcasing green chemistry's impact on pharmaceutical manufacturing.
Future Trends and Opportunities in Green Synthesis
Future trends in green chemistry emphasize the development of sustainable catalysts, renewable feedstocks, and energy-efficient processes that reduce hazardous waste and carbon footprints. Advances in enzymatic catalysis, microwave-assisted synthesis, and solvent-free reactions are opening new pathways for eco-friendly production. Emerging opportunities lie in integrating green synthesis with artificial intelligence to optimize reaction conditions and scale-up environmentally benign manufacturing.
Atom economy
Green chemistry prioritizes high atom economy to minimize waste and enhance efficiency, whereas conventional synthesis often results in lower atom economy due to the generation of excess by-products.
E-factor
Green chemistry significantly reduces the E-factor by minimizing waste generation compared to conventional synthesis methods.
Sustainable solvents
Green chemistry promotes sustainable solvents like water, supercritical CO2, and bio-based solvents that reduce toxicity and environmental impact compared to conventional synthesis using hazardous organic solvents.
Renewable feedstocks
Green chemistry prioritizes renewable feedstocks to reduce environmental impact, whereas conventional synthesis often relies on non-renewable, fossil-based materials.
Catalysis efficiency
Green chemistry utilizes highly efficient, sustainable catalysts that reduce energy consumption and waste compared to conventional synthesis catalysts with lower selectivity and higher environmental impact.
Toxicity reduction
Green chemistry significantly reduces toxicity by utilizing safer reagents and environmentally benign solvents compared to conventional synthesis, which often relies on hazardous chemicals and produces harmful byproducts.
Process intensification
Process intensification in green chemistry enhances reaction efficiency and sustainability by reducing energy consumption and waste compared to conventional synthesis methods.
Waste minimization
Green chemistry reduces waste generation through atom economy and safer reagents, whereas conventional synthesis often produces higher hazardous waste due to inefficient reactions and toxic solvents.
Energy-efficient pathways
Green chemistry employs energy-efficient pathways by utilizing renewable resources and catalytic processes, significantly reducing energy consumption compared to conventional synthesis methods reliant on high temperatures and harsh conditions.
Hazardous reagents
Green chemistry eliminates hazardous reagents by using safer, non-toxic alternatives, whereas conventional synthesis often relies on toxic, flammable, or environmentally damaging chemicals.
Green chemistry vs Conventional synthesis Infographic
 njnir.com
njnir.com