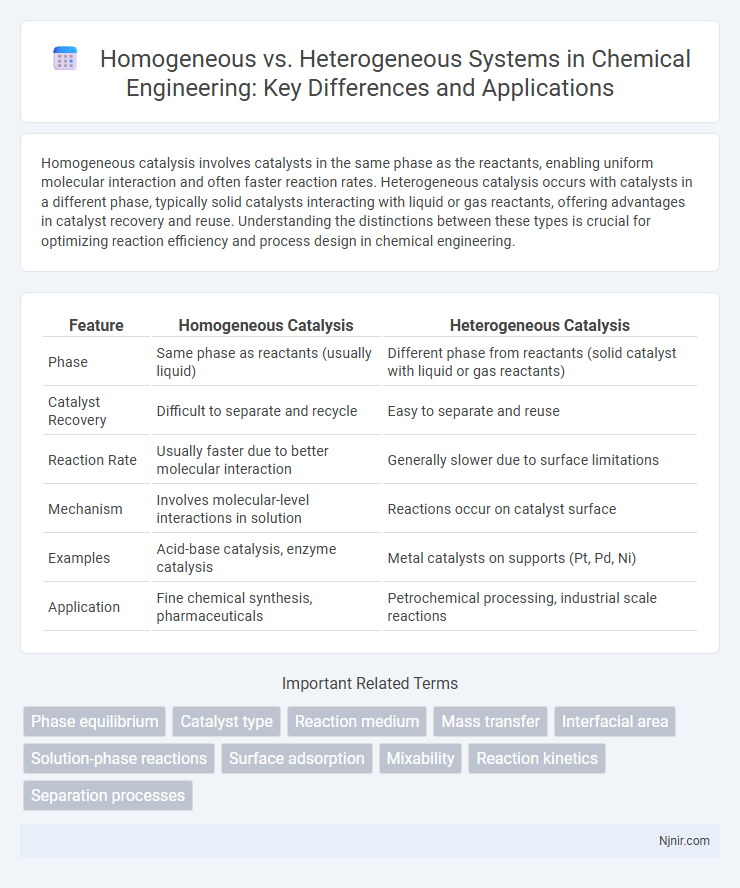
Homogeneous catalysis involves catalysts in the same phase as the reactants, enabling uniform molecular interaction and often faster reaction rates. Heterogeneous catalysis occurs with catalysts in a different phase, typically solid catalysts interacting with liquid or gas reactants, offering advantages in catalyst recovery and reuse. Understanding the distinctions between these types is crucial for optimizing reaction efficiency and process design in chemical engineering.
Table of Comparison
| Feature | Homogeneous Catalysis | Heterogeneous Catalysis |
|---|---|---|
| Phase | Same phase as reactants (usually liquid) | Different phase from reactants (solid catalyst with liquid or gas reactants) |
| Catalyst Recovery | Difficult to separate and recycle | Easy to separate and reuse |
| Reaction Rate | Usually faster due to better molecular interaction | Generally slower due to surface limitations |
| Mechanism | Involves molecular-level interactions in solution | Reactions occur on catalyst surface |
| Examples | Acid-base catalysis, enzyme catalysis | Metal catalysts on supports (Pt, Pd, Ni) |
| Application | Fine chemical synthesis, pharmaceuticals | Petrochemical processing, industrial scale reactions |
Introduction to Homogeneous and Heterogeneous Systems
Homogeneous systems consist of components that are uniform and identical in structure and function, enabling seamless integration and consistent performance across the system. Heterogeneous systems integrate diverse and distinct elements, often combining various hardware, software, or networks to leverage specialized capabilities and improve overall flexibility. Understanding the distinctions between homogeneous and heterogeneous systems is crucial for optimizing system architecture, resource allocation, and performance outcomes in computing and engineering contexts.
Fundamental Definitions and Key Differences
Homogeneous mixtures consist of uniformly distributed components with a single-phase appearance, such as salt dissolved in water, where the composition is consistent throughout. Heterogeneous mixtures feature distinct phases and visibly different substances, like sand mixed with iron filings, where components remain separate and identifiable. The key difference lies in the uniformity and phase distribution, with homogeneous mixtures being single-phase and indistinguishable, whereas heterogeneous mixtures are multi-phase and visibly diverse.
Molecular Interactions in Homogeneous and Heterogeneous Phases
Molecular interactions in homogeneous phases occur uniformly throughout a single phase, resulting in consistent intermolecular forces such as van der Waals interactions or hydrogen bonding within liquids or gases. In contrast, heterogeneous phases involve distinct interfaces between different phases, where molecular interactions differ significantly at boundaries, often leading to phenomena such as adsorption, surface tension, and interfacial energy variations. These differences critically influence material properties, reaction kinetics, and phase behavior in chemical systems.
Examples in Industrial Chemical Processes
In industrial chemical processes, homogeneous reactions occur when reactants and catalysts exist in the same phase, such as the acidic hydrolysis of esters where both the reactant and acid catalyst are in the liquid phase. Heterogeneous catalysis involves different phases, exemplified by the Haber-Bosch process for ammonia synthesis, where gaseous nitrogen and hydrogen react over a solid iron catalyst. These phase distinctions influence reaction efficiency, catalyst recovery, and process design in large-scale chemical manufacturing.
Catalyst Roles: Homogeneous vs Heterogeneous Catalysis
Homogeneous catalysis involves catalysts that exist in the same phase as the reactants, typically in solution, enabling uniform distribution and molecular-level interactions that enhance reaction rates and selectivity. Heterogeneous catalysis features solid catalysts interacting with gaseous or liquid reactants at the catalyst surface, facilitating reactions through adsorption, surface reactions, and desorption steps. The distinct physical states influence catalyst recovery, recyclability, and applicability in industrial processes such as petrochemical refining and pharmaceutical synthesis.
Kinetics and Mechanisms: Comparative Analysis
Homogeneous catalysis involves reactants and catalysts in the same phase, often resulting in uniform reaction kinetics and well-defined molecular mechanisms that facilitate precise mechanistic studies. Heterogeneous catalysis occurs at the interface of different phases, typically solid catalysts with gaseous or liquid reactants, characterized by complex kinetics influenced by surface adsorption, diffusion, and active site heterogeneity. Comparative analysis reveals that homogeneous systems generally exhibit faster reaction rates and simpler kinetic models, while heterogeneous systems offer advantages in catalyst recovery and durability despite more intricate mechanistic pathways.
Advantages and Limitations of Each System
Homogeneous systems offer advantages such as uniformity in design and ease of management, leading to simplified troubleshooting and consistent performance across all components. However, they face limitations including reduced flexibility and potential vulnerabilities to widespread failures due to identical system weaknesses. Heterogeneous systems provide enhanced adaptability, improved fault tolerance, and the ability to utilize diverse technologies, but they often involve increased complexity, higher maintenance costs, and challenges in integration and interoperability.
Application in Separation and Purification Techniques
Homogeneous catalysts operate in the same phase as reactants, enhancing selectivity and efficiency in separation and purification processes like liquid-liquid extraction and chromatography. Heterogeneous catalysts, present in a different phase, offer advantages in ease of separation and reusability, commonly applied in adsorption and filtration techniques. Selecting between homogeneous and heterogeneous methods depends on factors such as reaction conditions, product purity requirements, and catalyst recovery efficiency.
Environmental Impact and Sustainability Considerations
Homogeneous materials, characterized by uniform composition, often allow for easier recycling and resource recovery, thereby reducing environmental impact and supporting sustainability goals. In contrast, heterogeneous materials, composed of multiple diverse components, typically pose challenges in recycling processes, leading to increased waste and higher environmental burden. Sustainable material management favors homogeneous substances to minimize ecological footprint and promote circular economy practices.
Future Trends and Emerging Technologies
Future trends in homogeneous and heterogeneous computing reveal a growing reliance on heterogeneous architectures to optimize performance, energy efficiency, and scalability in AI, IoT, and edge computing. Emerging technologies, such as AI accelerators, neuromorphic chips, and specialized ASICs, drive the shift towards heterogeneous systems by integrating diverse processing units tailored for specific tasks. Advancements in software frameworks and interconnect standards further facilitate seamless collaboration between homogeneous and heterogeneous components, enabling more adaptive and efficient computing solutions.
Phase equilibrium
Phase equilibrium in homogeneous systems occurs within a single uniform phase, while in heterogeneous systems it involves multiple distinct phases coexisting and interacting.
Catalyst type
Homogeneous catalysts consist of active substances uniformly dispersed in the same phase as reactants, enhancing reaction rates through molecular-level interaction, while heterogeneous catalysts operate in a separate phase, typically solid catalysts interacting with gaseous or liquid reactants, enabling easier separation and reuse.
Reaction medium
Homogeneous reaction media consist of a single phase facilitating uniform reactant interaction, whereas heterogeneous reaction media involve multiple phases creating interfaces that influence reaction rates and mechanisms.
Mass transfer
Homogeneous mass transfer involves the movement of molecules within a single phase, while heterogeneous mass transfer occurs across the interface between two distinct phases, significantly impacting transfer rates and mechanisms.
Interfacial area
Homogeneous mixtures have minimal interfacial area due to uniform composition, while heterogeneous mixtures exhibit significant interfacial area between distinct phases, impacting reaction rates and material properties.
Solution-phase reactions
Homogeneous solution-phase reactions involve reactants and catalysts in the same phase, typically liquid, enhancing molecular interactions and reaction rates, whereas heterogeneous solution-phase reactions feature catalysts in a different phase, such as solid catalysts in liquid solutions, allowing easy separation and reuse but potentially slower reaction kinetics.
Surface adsorption
Surface adsorption efficiency varies between homogeneous catalysts, which provide uniform active sites, and heterogeneous catalysts, characterized by diverse surface properties influencing adsorption capacity and selectivity.
Mixability
Homogeneous mixtures exhibit uniform composition and high mixability at the molecular level, whereas heterogeneous mixtures consist of distinct components with limited mixability.
Reaction kinetics
Homogeneous reactions occur with reactants in the same phase, typically exhibiting faster reaction kinetics due to uniform molecular interactions, whereas heterogeneous reactions involve reactants in different phases, often displaying slower kinetics limited by surface area and diffusion rates.
Separation processes
Separation processes in homogeneous mixtures involve uniform phase separation like distillation, while heterogeneous mixtures require mechanical or physical methods such as filtration or sedimentation to separate distinct phases.
Homogeneous vs Heterogeneous Infographic
 njnir.com
njnir.com