Supercritical CO2 extraction offers a greener and more selective alternative to traditional solvent extraction by using CO2 above its critical temperature and pressure, ensuring efficient solute separation without toxic residues. This method preserves heat-sensitive compounds and reduces environmental impact through the elimination of hazardous solvents. Solvent extraction, while effective and widely used, often involves organic solvents that pose safety, toxicity, and disposal challenges, making supercritical CO2 extraction a preferred choice for sustainable chemical engineering processes.
Table of Comparison
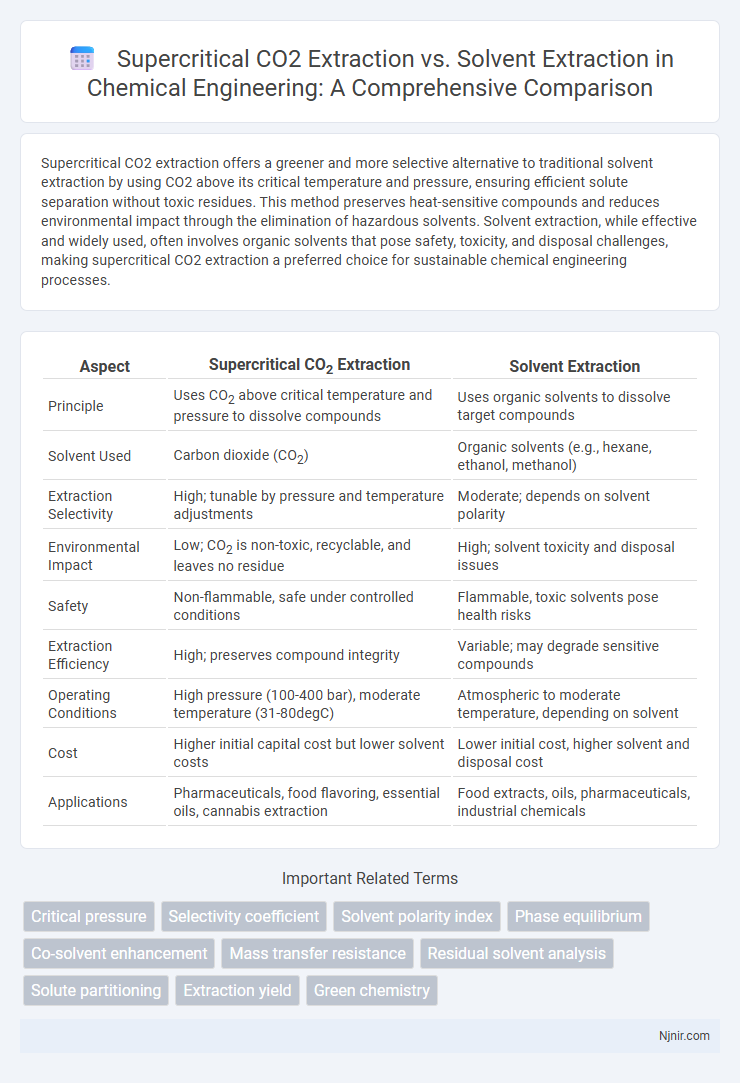
| Aspect | Supercritical CO2 Extraction | Solvent Extraction |
|---|---|---|
| Principle | Uses CO2 above critical temperature and pressure to dissolve compounds | Uses organic solvents to dissolve target compounds |
| Solvent Used | Carbon dioxide (CO2) | Organic solvents (e.g., hexane, ethanol, methanol) |
| Extraction Selectivity | High; tunable by pressure and temperature adjustments | Moderate; depends on solvent polarity |
| Environmental Impact | Low; CO2 is non-toxic, recyclable, and leaves no residue | High; solvent toxicity and disposal issues |
| Safety | Non-flammable, safe under controlled conditions | Flammable, toxic solvents pose health risks |
| Extraction Efficiency | High; preserves compound integrity | Variable; may degrade sensitive compounds |
| Operating Conditions | High pressure (100-400 bar), moderate temperature (31-80degC) | Atmospheric to moderate temperature, depending on solvent |
| Cost | Higher initial capital cost but lower solvent costs | Lower initial cost, higher solvent and disposal cost |
| Applications | Pharmaceuticals, food flavoring, essential oils, cannabis extraction | Food extracts, oils, pharmaceuticals, industrial chemicals |
Introduction to Extraction Techniques in Chemical Engineering
Supercritical CO2 extraction utilizes carbon dioxide at elevated temperature and pressure beyond its critical point, enabling selective and efficient separation of target compounds without residual solvents. Solvent extraction relies on organic solvents to dissolve specific components based on solubility differences, often requiring longer processing times and solvent removal steps. Supercritical CO2 extraction offers advantages in terms of environmental safety, product purity, and operational control, making it a preferred technique in chemical engineering applications for extracting bioactive compounds and essential oils.
Fundamentals of Supercritical CO2 Extraction
Supercritical CO2 extraction utilizes carbon dioxide above its critical temperature and pressure, where it exhibits unique solvation properties that enhance the selective extraction of compounds without leaving harmful residues. This method offers adjustable solvent strength by modulating pressure and temperature, enabling precise targeting of specific bioactive components. Compared to traditional solvent extraction, supercritical CO2 extraction is environmentally friendly, non-toxic, and produces high-purity extracts, making it a preferred choice in pharmaceuticals, nutraceuticals, and food industries.
Principles of Conventional Solvent Extraction
Conventional solvent extraction relies on the principle of solubility, where organic solvents dissolve target compounds from raw materials based on polarity and chemical affinity. The process involves mixing the solvent with the feedstock, allowing bioactive compounds to migrate into the solvent phase, followed by separation and solvent evaporation to recover the extract. This method is widely used for its simplicity and effectiveness but may leave solvent residues and lacks the tunable selectivity of supercritical CO2 extraction.
Comparative Extraction Efficiency: Supercritical CO2 vs Solvent Methods
Supercritical CO2 extraction offers higher selectivity and purity compared to traditional solvent extraction by using non-toxic CO2 at supercritical conditions, which penetrates plant matrices more effectively and preserves bioactive compounds. Solvent extraction often involves toxic organic solvents like hexane or ethanol, leading to potential solvent residues and lower extraction efficiency for thermolabile compounds. Overall, supercritical CO2 demonstrates superior extraction efficiency, minimal solvent waste, and better preservation of sensitive phytochemicals.
Environmental Impact and Sustainability Considerations
Supercritical CO2 extraction utilizes carbon dioxide at high pressure and temperature, producing a non-toxic, recyclable solvent with minimal environmental emissions, significantly reducing hazardous waste compared to traditional solvent extraction which relies on volatile organic compounds (VOCs) that contribute to air and water pollution. The closed-loop system of supercritical CO2 extraction enhances sustainability by minimizing solvent loss and energy consumption, while conventional solvent extraction often requires large quantities of petroleum-based solvents, increasing carbon footprint and disposal challenges. Sustainable manufacturing benefits from supercritical CO2's ability to extract bioactives efficiently without solvent residues, supporting eco-friendly product labeling and compliance with increasingly stringent environmental regulations.
Process Safety and Operational Risks
Supercritical CO2 extraction offers enhanced process safety by operating at lower temperatures and using non-flammable CO2, reducing fire and explosion hazards common in solvent extraction with volatile organic solvents such as hexane. The closed-loop system in supercritical CO2 extraction minimizes solvent emissions and worker exposure, significantly lowering operational risks related to toxicity and environmental contamination. In contrast, solvent extraction involves handling and storage of hazardous chemicals, increasing risks of leaks, spills, and fire, necessitating stringent safety protocols and ventilation systems to mitigate operational hazards.
Quality and Purity of Extracted Products
Supercritical CO2 extraction produces higher-quality and purer extracts due to its ability to selectively target specific compounds without leaving harmful solvent residues. Solvent extraction often results in impurities and potential contamination from residual solvents, which can compromise product safety and flavor. The non-toxic, environmentally friendly nature of CO2 also preserves the integrity and potency of delicate compounds better than traditional solvents.
Industrial Applications and Case Studies
Supercritical CO2 extraction offers a solvent-free, environmentally friendly alternative for industries like pharmaceuticals, cosmetics, and food processing, enabling high-purity compound isolation with minimal thermal degradation. Industrial case studies demonstrate its efficiency in extracting essential oils and bioactive compounds, yielding superior product quality compared to traditional solvent extraction, which relies on chemical solvents like hexane or ethanol and poses residual solvent concerns. While solvent extraction remains prevalent for bulk oil production due to lower capital costs, supercritical CO2 extraction is favored in high-value applications requiring precise selectivity and regulatory compliance.
Economic Analysis: Cost and Scalability
Supercritical CO2 extraction incurs higher initial capital expenditure due to specialized high-pressure equipment, yet offers lower operational costs through solvent recycling and reduced post-processing, enhancing long-term economic efficiency. Solvent extraction involves lower upfront investment but generates increased costs from solvent purchase, disposal, and potential regulatory compliance, limiting scalability for large-scale operations. Economically, supercritical CO2 extraction proves more scalable and cost-effective for high-purity products in industrial applications despite higher capital intensity.
Future Trends and Innovations in Extraction Technologies
Emerging trends in supercritical CO2 extraction highlight advancements in tunable pressure and temperature controls, enhancing selectivity and yield of bioactive compounds compared to traditional solvent extraction. Innovations such as integrating ultrasonic and microwave pretreatments with supercritical CO2 technology improve mass transfer rates and reduce extraction times while maintaining eco-friendly profiles. Future development focuses on scalable continuous flow systems and hybrid extraction methods to maximize efficiency, sustainability, and purity in nutraceutical and pharmaceutical industries.
Critical pressure
Supercritical CO2 extraction operates at a critical pressure of approximately 73.8 bar, enabling selective and efficient compound isolation, while solvent extraction typically uses lower pressures but relies on chemical solvents that may leave residues.
Selectivity coefficient
Supercritical CO2 extraction exhibits a higher selectivity coefficient than solvent extraction by precisely targeting specific compounds based on pressure and temperature adjustments, enhancing purity and yield of desired extracts.
Solvent polarity index
Solvent extraction efficiency is heavily influenced by the solvent polarity index, whereas supercritical CO2 extraction relies on tunable solvating power without traditional polarity constraints.
Phase equilibrium
Supercritical CO2 extraction offers precise phase equilibrium control enabling superior selectivity and efficiency compared to traditional solvent extraction methods relying on liquid-liquid phase equilibrium.
Co-solvent enhancement
Supercritical CO2 extraction with co-solvent enhancement improves yield and selectivity compared to traditional solvent extraction by increasing solubility of polar compounds and reducing solvent residues.
Mass transfer resistance
Supercritical CO2 extraction exhibits lower mass transfer resistance compared to solvent extraction, enhancing the efficiency and selectivity of bioactive compound isolation.
Residual solvent analysis
Supercritical CO2 extraction leaves negligible residual solvents compared to traditional solvent extraction, which often requires rigorous residual solvent analysis to ensure product safety and regulatory compliance.
Solute partitioning
Supercritical CO2 extraction enhances solute partitioning by selectively dissolving target compounds in a tunable solvent environment, whereas solvent extraction relies on fixed polarity solvents, often leading to less efficient and less selective solute partitioning.
Extraction yield
Supercritical CO2 extraction offers higher extraction yield with greater purity and selectivity compared to traditional solvent extraction methods.
Green chemistry
Supercritical CO2 extraction offers a greener chemistry approach than traditional solvent extraction by using non-toxic, recyclable CO2 that minimizes hazardous solvent waste and reduces environmental impact.
Supercritical CO2 extraction vs Solvent extraction Infographic
 njnir.com
njnir.com