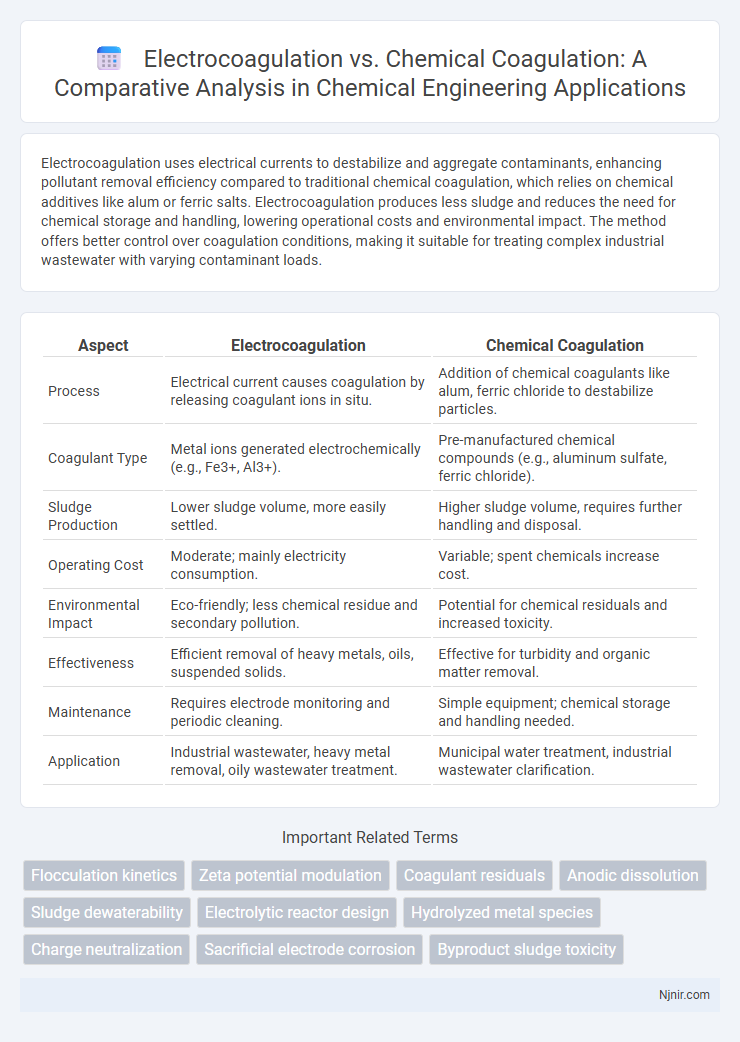
Electrocoagulation uses electrical currents to destabilize and aggregate contaminants, enhancing pollutant removal efficiency compared to traditional chemical coagulation, which relies on chemical additives like alum or ferric salts. Electrocoagulation produces less sludge and reduces the need for chemical storage and handling, lowering operational costs and environmental impact. The method offers better control over coagulation conditions, making it suitable for treating complex industrial wastewater with varying contaminant loads.
Table of Comparison
| Aspect | Electrocoagulation | Chemical Coagulation |
|---|---|---|
| Process | Electrical current causes coagulation by releasing coagulant ions in situ. | Addition of chemical coagulants like alum, ferric chloride to destabilize particles. |
| Coagulant Type | Metal ions generated electrochemically (e.g., Fe3+, Al3+). | Pre-manufactured chemical compounds (e.g., aluminum sulfate, ferric chloride). |
| Sludge Production | Lower sludge volume, more easily settled. | Higher sludge volume, requires further handling and disposal. |
| Operating Cost | Moderate; mainly electricity consumption. | Variable; spent chemicals increase cost. |
| Environmental Impact | Eco-friendly; less chemical residue and secondary pollution. | Potential for chemical residuals and increased toxicity. |
| Effectiveness | Efficient removal of heavy metals, oils, suspended solids. | Effective for turbidity and organic matter removal. |
| Maintenance | Requires electrode monitoring and periodic cleaning. | Simple equipment; chemical storage and handling needed. |
| Application | Industrial wastewater, heavy metal removal, oily wastewater treatment. | Municipal water treatment, industrial wastewater clarification. |
Introduction to Coagulation in Water Treatment
Coagulation in water treatment involves destabilizing suspended particles to facilitate their aggregation and removal. Electrocoagulation uses electrical current to generate coagulants in situ, producing metal hydroxides that capture impurities, while chemical coagulation relies on adding chemical coagulants like alum or ferric chloride. Electrocoagulation offers advantages in reducing chemical sludge and improving contaminant removal efficiency compared to traditional chemical coagulation methods.
Fundamentals of Chemical Coagulation
Chemical coagulation relies on adding chemical coagulants, such as aluminum sulfate or ferric chloride, to destabilize colloidal particles through charge neutralization and sweep flocculation. This process involves rapid mixing to disperse the coagulant, followed by slow mixing to encourage floc formation and particle agglomeration. Unlike electrocoagulation, which uses electric current to generate coagulant species in situ, chemical coagulation depends entirely on the dosage and type of chemicals added for effective contaminant removal.
Principles of Electrocoagulation Technology
Electrocoagulation technology operates by applying an electric current to sacrificial metal electrodes, typically aluminum or iron, generating metal hydroxide flocs that destabilize and aggregate contaminants in water. This process promotes in-situ production of coagulants, minimizing the need for chemical additives used in conventional chemical coagulation methods. Electrocoagulation enhances removal efficiency of suspended solids, heavy metals, and pathogens through combined electrochemical reactions such as oxidation, reduction, and electroflotation.
Comparative Mechanisms: Chemical vs. Electrocoagulation
Electrocoagulation utilizes electrical current to dissolve sacrificial anodes, generating coagulant ions in situ, whereas chemical coagulation relies on the direct addition of chemical coagulants like alum or ferric chloride. The electrochemical process promotes efficient destabilization and aggregation of suspended particles through electrochemical reactions, while chemical coagulation depends on chemical hydrolysis and charge neutralization. Electrocoagulation offers advantages in reducing sludge production and chemical consumption compared to traditional chemical coagulation methods.
Efficiency in Contaminant Removal
Electrocoagulation demonstrates superior efficiency in contaminant removal by generating coagulants in situ, enabling rapid destabilization of suspended particles and heavy metals such as lead and arsenic. Chemical coagulation relies on externally added chemicals like alum or ferric chloride, which may require precise dosing and longer settling times to achieve comparable removal rates. Studies indicate electrocoagulation can reduce turbidity and chemical oxygen demand (COD) by over 90%, outperforming traditional chemical methods in both effectiveness and environmental impact.
Environmental Impact and Sustainability
Electrocoagulation offers a more environmentally sustainable alternative to chemical coagulation by reducing chemical usage and minimizing sludge generation, which lowers disposal impacts. The process employs electrical currents to destabilize contaminants, leading to less secondary pollution and improved biodegradability of treated water. In contrast, chemical coagulation relies heavily on coagulant chemicals like aluminum and iron salts, whose production and residuals can pose significant environmental risks and contribute to higher operational costs.
Operational and Maintenance Requirements
Electrocoagulation requires less chemical handling and storage, reducing operational complexity and environmental risks compared to chemical coagulation, which relies on precise dosing of coagulants like alum or ferric chloride. Maintenance of electrocoagulation systems involves periodic electrode cleaning and replacement to prevent passivation, whereas chemical coagulation demands regular equipment calibration and sludge handling to manage residuals. Energy consumption is a critical operational factor for electrocoagulation, influencing cost and system efficiency, while chemical coagulation requires ongoing supply chain management for chemicals.
Cost Analysis: Capital and Operational Expenses
Electrocoagulation typically requires higher initial capital expenses due to specialized equipment like power supplies and electrodes, whereas chemical coagulation involves lower upfront costs mainly associated with chemical storage and dosing systems. Operational expenses for electrocoagulation can be lower over time, benefiting from reduced chemical consumption and sludge generation, but energy costs can significantly impact overall expenses. Chemical coagulation's ongoing costs are dominated by chemical procurement and handling, which may fluctuate based on market prices and require additional expenditure for sludge disposal.
Case Studies and Industrial Applications
Electrocoagulation demonstrates superior performance in heavy metal removal and organic pollutant degradation across diverse industrial wastewater treatment case studies, with notable efficiency in textile and pharmaceutical effluents. Chemical coagulation, widely applied in municipal water treatment and paper mill effluent management, relies on aluminum and iron salts but often produces higher sludge volumes and requires pH adjustments. Industrial applications reveal electrocoagulation's advantages in operational cost reduction and lower secondary pollution, whereas chemical coagulation remains prevalent due to established protocols and reagent availability.
Future Trends and Innovations in Coagulation Techniques
Electrocoagulation is emerging with future trends emphasizing energy efficiency and automation through IoT integration, offering real-time process monitoring for enhanced water treatment. Innovations in chemical coagulation focus on sustainable, biodegradable coagulants derived from natural sources, reducing environmental impact and sludge volume. Hybrid systems combining electrocoagulation and chemical methods are gaining traction, optimizing contaminant removal while minimizing operational costs and chemical usage.
Flocculation kinetics
Electrocoagulation accelerates flocculation kinetics by generating coagulant in situ and producing buoyant flocs, whereas chemical coagulation relies on externally added chemicals that form denser flocs with slower aggregation rates.
Zeta potential modulation
Electrocoagulation effectively modulates zeta potential by generating in-situ coagulants and altering particle surface charges, resulting in enhanced destabilization compared to traditional chemical coagulation methods.
Coagulant residuals
Electrocoagulation produces lower coagulant residuals compared to chemical coagulation, reducing secondary pollution and minimizing the need for additional treatment.
Anodic dissolution
Electrocoagulation relies on anodic dissolution of sacrificial anodes to generate metal ions in situ for contaminant removal, whereas chemical coagulation involves direct addition of external coagulant chemicals without anodic material consumption.
Sludge dewaterability
Electrocoagulation produces sludge with higher dewaterability and lower moisture content compared to chemical coagulation, enhancing sludge handling and reducing disposal costs.
Electrolytic reactor design
Electrolytic reactor design in electrocoagulation optimizes electrode configuration, current density, and flow dynamics to enhance contaminant removal efficiency compared to chemical coagulation.
Hydrolyzed metal species
Electrocoagulation produces hydrolyzed metal species in situ with higher reactivity and stability compared to the externally added metal salts in chemical coagulation, enhancing contaminant removal efficiency.
Charge neutralization
Electrocoagulation achieves charge neutralization by generating coagulant ions in situ through electrical current, enhancing particle destabilization more efficiently than traditional chemical coagulation methods.
Sacrificial electrode corrosion
Electrocoagulation utilizes sacrificial electrodes such as aluminum or iron that corrode to release coagulant ions directly into water, offering controlled metal ion dosage and reduced chemical usage compared to chemical coagulation.
Byproduct sludge toxicity
Electrocoagulation generates significantly less toxic sludge compared to chemical coagulation, reducing hazardous byproducts and environmental disposal risks.
Electrocoagulation vs Chemical coagulation Infographic
 njnir.com
njnir.com