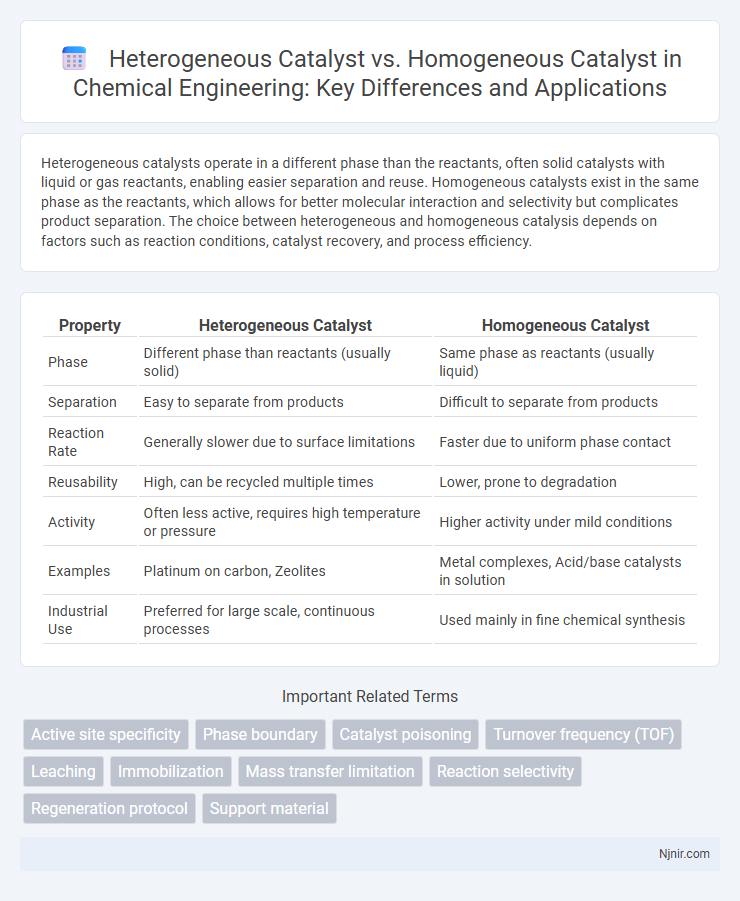
Heterogeneous catalysts operate in a different phase than the reactants, often solid catalysts with liquid or gas reactants, enabling easier separation and reuse. Homogeneous catalysts exist in the same phase as the reactants, which allows for better molecular interaction and selectivity but complicates product separation. The choice between heterogeneous and homogeneous catalysis depends on factors such as reaction conditions, catalyst recovery, and process efficiency.
Table of Comparison
| Property | Heterogeneous Catalyst | Homogeneous Catalyst |
|---|---|---|
| Phase | Different phase than reactants (usually solid) | Same phase as reactants (usually liquid) |
| Separation | Easy to separate from products | Difficult to separate from products |
| Reaction Rate | Generally slower due to surface limitations | Faster due to uniform phase contact |
| Reusability | High, can be recycled multiple times | Lower, prone to degradation |
| Activity | Often less active, requires high temperature or pressure | Higher activity under mild conditions |
| Examples | Platinum on carbon, Zeolites | Metal complexes, Acid/base catalysts in solution |
| Industrial Use | Preferred for large scale, continuous processes | Used mainly in fine chemical synthesis |
Introduction to Catalysis in Chemical Engineering
Heterogeneous catalysts operate in a different phase than the reactants, typically solids interacting with gas or liquid reactants, enabling easy separation and reuse in chemical engineering processes. Homogeneous catalysts share the same phase as reactants, often liquid, providing uniform active sites but complicating separation and recovery. Catalysis in chemical engineering optimizes reaction rates and selectivity by leveraging these catalyst types for applications ranging from petrochemical refining to pharmaceutical synthesis.
Defining Heterogeneous and Homogeneous Catalysts
Heterogeneous catalysts exist in a different phase than the reactants, typically solid catalysts interacting with liquid or gas-phase reactants, facilitating surface reactions. Homogeneous catalysts share the same phase, usually a solution phase, enabling molecular-level interactions and uniform catalyst distribution. Key distinctions include catalyst separation ease and reaction mechanism, with heterogeneous catalysts offering straightforward recovery and homogeneous catalysts providing high selectivity.
Mechanistic Differences in Catalytic Processes
Heterogeneous catalysts operate through surface adsorption where reactants bind to active sites on solid materials, facilitating bond breaking and formation via multiple elementary steps; their mechanism often includes diffusion limitations and surface intermediates. Homogeneous catalysts function in the same phase as reactants, enabling molecular-level interactions and well-defined transition states, often proceeding through coordinatively unsaturated metal complexes or organocatalysts with precise ligand environments. Mechanistically, heterogeneous catalysis typically involves Langmuir-Hinshelwood or Eley-Rideal pathways, while homogeneous catalysis proceeds through catalytic cycles involving ligand exchange and redox changes.
Advantages of Heterogeneous Catalysts
Heterogeneous catalysts offer significant advantages such as ease of separation from reaction mixtures due to their solid state, enabling straightforward catalyst recovery and reuse, which improves process efficiency and reduces costs. Their structural stability under diverse reaction conditions allows for enhanced durability and consistent catalytic performance over extended periods. The availability of a wide range of support materials further facilitates catalyst design, promoting higher selectivity and activity in industrial applications.
Benefits of Homogeneous Catalysts
Homogeneous catalysts offer superior selectivity and activity due to their intimate interaction with reactants in the same phase, often liquid, enhancing reaction control and efficiency. Their molecular-level uniformity facilitates precise catalyst design and tuning, enabling tailored reaction pathways and minimized byproduct formation. Moreover, homogeneous catalysts typically operate under milder conditions, reducing energy consumption and improving overall process sustainability in chemical synthesis.
Industrial Applications and Case Studies
Heterogeneous catalysts, widely used in industrial applications such as petroleum refining and ammonia synthesis, offer advantages in catalyst recovery and stability due to their solid phase, enhancing process efficiency and scalability. Homogeneous catalysts dominate in fine chemical production and pharmaceutical synthesis, providing superior selectivity and reaction control but often require complex separation techniques post-reaction. Case studies in methanol production highlight heterogeneous catalyst efficiency in continuous flow systems, whereas hydroformylation reactions exemplify homogeneous catalysts' precision in forming high-value intermediates under mild conditions.
Catalyst Separation and Recycling Techniques
Heterogeneous catalysts are easily separated from reaction mixtures through filtration or centrifugation due to their solid-phase nature, enabling efficient recycling and reuse in industrial processes. Homogeneous catalysts, often dissolved in the same phase as reactants, require advanced separation techniques such as liquid-liquid extraction, membrane dialysis, or chromatographic methods to achieve catalyst recovery and recycling. Innovations in immobilizing homogeneous catalysts on solid supports or designing bifunctional catalysts improve separation efficiency and sustainability in catalytic applications.
Reaction Conditions and Operational Efficiency
Heterogeneous catalysts operate under a wider range of reaction conditions, including high temperatures and pressures, due to their solid-state nature, which enhances durability and ease of separation from reaction mixtures. Homogeneous catalysts, typically in liquid form, require milder conditions and precise control of temperature and pH to maintain solubility and catalytic activity, impacting operational efficiency. The ease of catalyst recovery and reuse is generally higher with heterogeneous catalysts, contributing to better process scalability and reduced operational costs.
Environmental Impact and Sustainability
Heterogeneous catalysts, often solid substances, facilitate easier separation and reuse, reducing waste and minimizing environmental contamination compared to homogeneous catalysts, which are typically liquid and more challenging to recover. The durability and recyclability of heterogeneous catalysts contribute significantly to sustainable industrial processes by lowering resource consumption and energy usage. In contrast, homogeneous catalysts, while offering high selectivity, often require complex recycling methods that can increase environmental burdens and operational costs.
Future Trends in Catalyst Development
Future trends in catalyst development emphasize the integration of heterogeneous catalysts with nanotechnology to enhance surface area and active site accessibility, improving efficiency and selectivity in industrial processes. Homogeneous catalysts are being advanced through the design of modular ligand frameworks that allow precise control over catalytic activity and enable more sustainable, atom-economical reactions. Hybrid catalytic systems combining the scalability and recyclability of heterogeneous catalysts with the high specificity of homogeneous catalysts are emerging as a promising approach for next-generation sustainable chemical manufacturing.
Active site specificity
Heterogeneous catalysts exhibit active site specificity primarily on solid surfaces with distinct adsorption sites, whereas homogeneous catalysts offer uniform active sites within a single-phase solution, enabling precise molecular interactions.
Phase boundary
Heterogeneous catalysts operate at the solid-liquid or solid-gas phase boundary facilitating surface reactions, while homogeneous catalysts function within a single phase, typically liquid, enabling uniform molecular interactions.
Catalyst poisoning
Heterogeneous catalysts are more resistant to catalyst poisoning due to their solid surface structure, whereas homogeneous catalysts often suffer from rapid deactivation because their molecular catalysts are more susceptible to binding by impurities.
Turnover frequency (TOF)
Heterogeneous catalysts typically exhibit lower turnover frequencies (TOF) compared to homogeneous catalysts due to limited active site accessibility despite offering easier separation and reuse.
Leaching
Heterogeneous catalysts exhibit lower leaching rates compared to homogeneous catalysts, enhancing catalyst stability and recyclability in chemical reactions.
Immobilization
Immobilization enhances the reusability and separation efficiency of heterogeneous catalysts by anchoring active sites on solid supports, whereas homogeneous catalysts, dissolved in the reaction medium, often lack straightforward immobilization methods, complicating catalyst recovery and recycling.
Mass transfer limitation
Heterogeneous catalysts often face mass transfer limitations due to phase boundaries restricting reactant diffusion, whereas homogeneous catalysts exhibit minimal mass transfer resistance as they operate within a single phase.
Reaction selectivity
Heterogeneous catalysts provide higher reaction selectivity due to their distinct surface active sites and phase separations, while homogeneous catalysts offer uniform active sites that can lead to more controlled but sometimes less selective reactions.
Regeneration protocol
Heterogeneous catalysts enable simpler regeneration protocols through physical separation and surface treatment, while homogeneous catalysts require complex chemical methods such as solvent extraction or precipitation for effective regeneration.
Support material
Heterogeneous catalysts rely on solid support materials such as silica, alumina, or zeolites to enhance catalyst stability and surface area, whereas homogeneous catalysts typically lack solid supports and operate entirely in solution, facilitating uniform catalytic activity but complicating separation.
Heterogeneous catalyst vs Homogeneous catalyst Infographic
 njnir.com
njnir.com