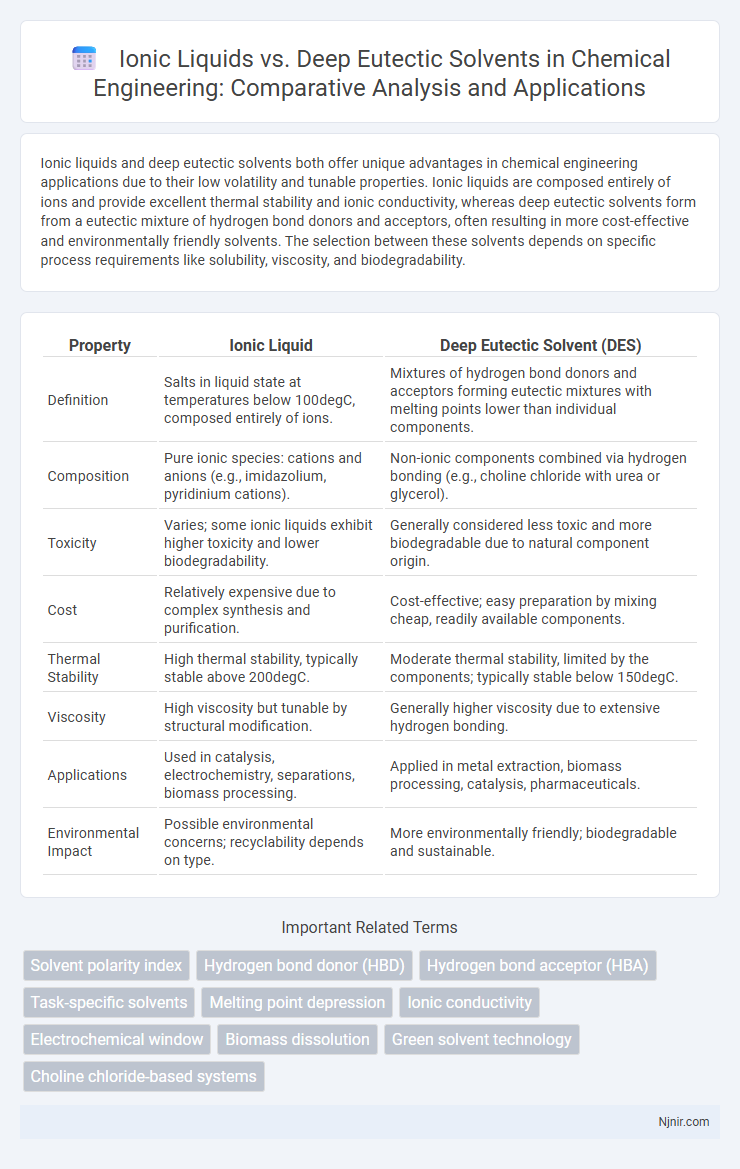
Ionic liquids and deep eutectic solvents both offer unique advantages in chemical engineering applications due to their low volatility and tunable properties. Ionic liquids are composed entirely of ions and provide excellent thermal stability and ionic conductivity, whereas deep eutectic solvents form from a eutectic mixture of hydrogen bond donors and acceptors, often resulting in more cost-effective and environmentally friendly solvents. The selection between these solvents depends on specific process requirements like solubility, viscosity, and biodegradability.
Table of Comparison
| Property | Ionic Liquid | Deep Eutectic Solvent (DES) |
|---|---|---|
| Definition | Salts in liquid state at temperatures below 100degC, composed entirely of ions. | Mixtures of hydrogen bond donors and acceptors forming eutectic mixtures with melting points lower than individual components. |
| Composition | Pure ionic species: cations and anions (e.g., imidazolium, pyridinium cations). | Non-ionic components combined via hydrogen bonding (e.g., choline chloride with urea or glycerol). |
| Toxicity | Varies; some ionic liquids exhibit higher toxicity and lower biodegradability. | Generally considered less toxic and more biodegradable due to natural component origin. |
| Cost | Relatively expensive due to complex synthesis and purification. | Cost-effective; easy preparation by mixing cheap, readily available components. |
| Thermal Stability | High thermal stability, typically stable above 200degC. | Moderate thermal stability, limited by the components; typically stable below 150degC. |
| Viscosity | High viscosity but tunable by structural modification. | Generally higher viscosity due to extensive hydrogen bonding. |
| Applications | Used in catalysis, electrochemistry, separations, biomass processing. | Applied in metal extraction, biomass processing, catalysis, pharmaceuticals. |
| Environmental Impact | Possible environmental concerns; recyclability depends on type. | More environmentally friendly; biodegradable and sustainable. |
Introduction to Ionic Liquids and Deep Eutectic Solvents
Ionic liquids are salts with melting points below 100degC, composed entirely of ions, exhibiting unique properties such as low volatility, high thermal stability, and tunable solubility, which make them valuable in green chemistry and electrochemical applications. Deep eutectic solvents (DES) are mixtures of hydrogen bond donors and acceptors that form a eutectic with a melting point significantly lower than individual components, offering biodegradable, cost-effective, and easy-to-prepare alternatives to ionic liquids. Both solvents provide environmentally friendly solutions for catalysis, separation processes, and materials synthesis, but DES typically offer greater simplicity and lower toxicity compared to conventional ionic liquids.
Chemical Structure and Composition
Ionic liquids consist of discrete ions, typically composed of bulky organic cations and various inorganic or organic anions, resulting in low melting points and high thermal stability. Deep eutectic solvents (DES) form through hydrogen bond interactions between a hydrogen bond donor and acceptor, creating a eutectic mixture with a melting point significantly lower than individual components. The chemical structure of ionic liquids involves ionic bonds between charged species, whereas DES rely predominantly on supramolecular interactions and hydrogen bonding for solvent formation.
Synthesis Methods and Scalability
Ionic liquids are synthesized through controlled quaternization reactions followed by anion exchange, allowing for precise tailoring of physicochemical properties, while their scalability is often limited by complex purification and high production costs. Deep eutectic solvents (DES) are produced via simple mixing of hydrogen bond donors and acceptors at moderate temperatures without needing additional purification steps, offering more cost-effective and scalable production suitable for industrial applications. The straightforward synthesis and availability of biodegradable components make DES more favorable for large-scale and environmentally friendly processes compared to traditional ionic liquids.
Physical and Chemical Properties Comparison
Ionic liquids exhibit low volatility, high thermal stability, and excellent ionic conductivity, making them suitable for a range of electrochemical applications, whereas deep eutectic solvents (DES) generally have higher viscosity and lower conductivity but offer biodegradable and less toxic profiles. Chemically, ionic liquids consist of bulky, asymmetric ions providing tunable polarity and solvating abilities, while DES are formed by mixing hydrogen bond donors and acceptors, leading to strong hydrogen bonding networks that influence their melting points and solvation characteristics. Both solvent classes demonstrate unique physicochemical properties such as melting point suppression and solvation versatility, but DES typically exhibit greater environmental compatibility and cost-effectiveness compared to ionic liquids.
Solubility Profiles and Tunability
Ionic liquids exhibit highly tunable solubility profiles due to their customizable cation-anion combinations, enabling dissolution of a wide range of organic and inorganic compounds. Deep eutectic solvents provide adjustable solubility characteristics through variation in hydrogen bond donor and acceptor components, offering a more environmentally friendly and cost-effective alternative. The tunability of both solvent types facilitates targeted applications in separation processes, catalysis, and biomass processing by optimizing solubility parameters for specific solutes.
Applications in Chemical Engineering Processes
Ionic liquids exhibit remarkable thermal stability and tunable solvation properties, making them ideal for catalysis, separation, and electrochemical applications in chemical engineering. Deep eutectic solvents offer cost-effective, biodegradable alternatives with comparable solvation capabilities, widely used in biomass processing, metal extraction, and green synthesis. Both solvents enhance reaction efficiency and selectivity, with ionic liquids favored for high-performance industrial processes and deep eutectic solvents preferred in sustainable and environmentally friendly applications.
Environmental Impact and Biodegradability
Ionic liquids often exhibit low volatility and high thermal stability but can pose environmental risks due to their potential toxicity and persistence in ecosystems. Deep eutectic solvents (DESs) are generally considered more environmentally friendly because they are typically composed of biodegradable, natural components that reduce ecotoxicity and enhance biodegradability. Research indicates that DESs offer a greener alternative with improved biodegradation rates and lower environmental impact compared to many conventional ionic liquids.
Cost Analysis and Economic Feasibility
Ionic liquids generally exhibit higher synthesis and purification costs due to complex alkylation and anion exchange processes, whereas deep eutectic solvents (DES) leverage abundant, low-cost components like choline chloride and organic acids, significantly reducing raw material expenses. Economic feasibility of DES is further enhanced by their facile preparation, lower toxicity, and biodegradability, which minimizes environmental compliance costs compared to the sometimes toxic and less biodegradable ionic liquids. Cost-benefit analysis indicates DES offer a more scalable and sustainable solvent alternative in industrial applications where budget constraints and green chemistry principles are prioritized.
Toxicity and Safety Considerations
Ionic liquids generally exhibit low volatility and high thermal stability but may pose toxicity risks depending on their cation-anion combinations, affecting environmental and human safety. Deep eutectic solvents (DESs) are often considered safer alternatives due to their biodegradable components and lower toxicity profiles, making them more environmentally friendly for industrial and pharmaceutical applications. Safety considerations necessitate thorough toxicity assessments of both solvents, as variations in chemical composition significantly influence their ecological impact and usability in green chemistry.
Future Trends and Research Directions
Future research on ionic liquids emphasizes designing task-specific solvents with enhanced biocompatibility and reduced toxicity for sustainable industrial applications. Deep eutectic solvents (DES) are gaining attention for their low-cost, biodegradable, and tunable properties, driving innovations in green chemistry and materials science. Emerging trends focus on hybrid systems combining ionic liquids and DES to optimize solvent performance in energy storage, catalysis, and pharmaceutical formulations.
Solvent polarity index
Ionic liquids typically exhibit a wide range of solvent polarity indices often higher than deep eutectic solvents, which generally have lower and more tunable polarity values influencing solvation capabilities.
Hydrogen bond donor (HBD)
Hydrogen bond donors (HBDs) in ionic liquids typically involve cationic species with labile protons, while deep eutectic solvents predominantly feature molecular components such as urea or glycerol that form extensive hydrogen bonding networks, influencing their physicochemical properties differently.
Hydrogen bond acceptor (HBA)
Ionic liquids typically use halide or bulky anions as hydrogen bond acceptors (HBAs), while deep eutectic solvents commonly feature metal salts or quaternary ammonium salts as more flexible and tunable HBAs, influencing their solvation and reactivity properties.
Task-specific solvents
Task-specific ionic liquids exhibit tailored physicochemical properties enhancing selectivity and efficiency, whereas deep eutectic solvents provide cost-effective, biodegradable alternatives with tunable polarity for specialized catalytic and extraction processes.
Melting point depression
Deep eutectic solvents exhibit greater melting point depression than ionic liquids due to their unique hydrogen-bonding networks that disrupt lattice structures more effectively.
Ionic conductivity
Ionic liquids exhibit higher ionic conductivity compared to deep eutectic solvents due to their lower viscosity and better ion mobility.
Electrochemical window
Ionic liquids typically exhibit wider electrochemical windows ranging from 4 to 6 V compared to deep eutectic solvents, which generally show narrower windows around 2 to 3 V, making ionic liquids more suitable for high-voltage electrochemical applications.
Biomass dissolution
Ionic liquids exhibit superior biomass dissolution efficiency compared to deep eutectic solvents due to their customizable ionic structures and stronger hydrogen-bonding capabilities.
Green solvent technology
Ionic liquids and deep eutectic solvents, both pivotal in green solvent technology, offer tunable properties and low volatility, with deep eutectic solvents often providing more cost-effective and biodegradable alternatives for sustainable industrial applications.
Choline chloride-based systems
Choline chloride-based deep eutectic solvents exhibit lower toxicity, cost-effectiveness, and easier synthesis compared to ionic liquids, making them advantageous for sustainable chemical processes.
Ionic liquid vs Deep eutectic solvent Infographic
 njnir.com
njnir.com