Thermochemical conversion involves the use of high temperatures to break down biomass into useful energy products through processes such as pyrolysis, gasification, and combustion, facilitating rapid transformation with high energy density outputs. Biochemical conversion relies on microorganisms or enzymes to biologically decompose organic material into biofuels like ethanol, emphasizing milder conditions and specificity in product formation. Both methods offer distinct advantages in biomass-to-energy conversion, with thermochemical processes favoring faster throughput and broader feedstock compatibility, while biochemical routes provide targeted biofuel production with potentially lower environmental impact.
Table of Comparison
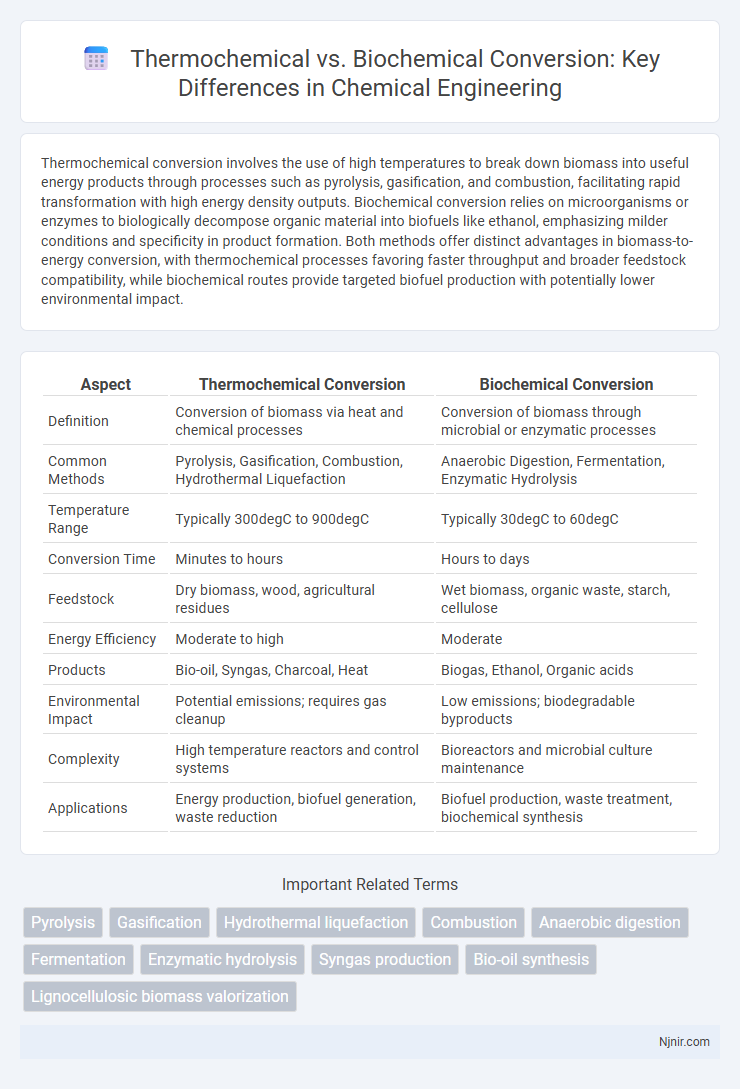
| Aspect | Thermochemical Conversion | Biochemical Conversion |
|---|---|---|
| Definition | Conversion of biomass via heat and chemical processes | Conversion of biomass through microbial or enzymatic processes |
| Common Methods | Pyrolysis, Gasification, Combustion, Hydrothermal Liquefaction | Anaerobic Digestion, Fermentation, Enzymatic Hydrolysis |
| Temperature Range | Typically 300degC to 900degC | Typically 30degC to 60degC |
| Conversion Time | Minutes to hours | Hours to days |
| Feedstock | Dry biomass, wood, agricultural residues | Wet biomass, organic waste, starch, cellulose |
| Energy Efficiency | Moderate to high | Moderate |
| Products | Bio-oil, Syngas, Charcoal, Heat | Biogas, Ethanol, Organic acids |
| Environmental Impact | Potential emissions; requires gas cleanup | Low emissions; biodegradable byproducts |
| Complexity | High temperature reactors and control systems | Bioreactors and microbial culture maintenance |
| Applications | Energy production, biofuel generation, waste reduction | Biofuel production, waste treatment, biochemical synthesis |
Overview of Thermochemical and Biochemical Conversion
Thermochemical conversion involves the use of heat to transform biomass into fuels, chemicals, and energy products through processes such as pyrolysis, gasification, and combustion, operating at high temperatures without oxygen or with limited oxygen. Biochemical conversion relies on microorganisms and enzymes to break down organic materials via fermentation or anaerobic digestion, producing biofuels like ethanol and biogas under milder temperature and pressure conditions. Both methods serve as sustainable alternatives for bioenergy production, with thermochemical processes favoring diverse feedstocks and rapid conversion, while biochemical methods emphasize specificity and compatibility with wet biomass.
Fundamental Principles of Thermochemical Conversion
Thermochemical conversion relies on high-temperature processes such as pyrolysis, gasification, and combustion to break down biomass into valuable fuels, gases, and char through chemical reactions driven by heat. This method involves decomposing organic materials in the absence or presence of limited oxygen, enabling the transformation of solid biomass into syngas, bio-oil, or biochar. The fundamental principle centers on thermal energy inducing molecular fragmentation and recombination, promoting efficient energy recovery from diverse feedstocks.
Core Concepts of Biochemical Conversion
Biochemical conversion involves the use of microorganisms and enzymes to break down organic materials into biofuels such as ethanol and biogas, relying primarily on processes like fermentation and anaerobic digestion. Key microorganisms include bacteria, yeast, and fungi, which metabolize carbohydrates into energy-rich compounds under controlled conditions. This method emphasizes mild operating temperatures, enzyme specificity, and substrate compatibility for efficient biomass transformation into renewable energy sources.
Feedstock Suitability and Preparation
Thermochemical conversion processes, such as pyrolysis and gasification, are well-suited for a wide range of feedstocks including lignocellulosic biomass, agricultural residues, and waste materials due to their tolerance for moisture content and particle size variability. Biochemical conversion techniques like anaerobic digestion and enzymatic hydrolysis require feedstocks with lower lignin content and often demand intensive pretreatment steps including size reduction, washing, and enzymatic or acid hydrolysis to enhance substrate accessibility. Feedstock preparation for biochemical methods involves moisture adjustment and contaminant removal to optimize microbial or enzymatic activity, while thermochemical methods generally accommodate less refined feedstocks but may require drying for efficient thermal processing.
Process Efficiency and Product Yields
Thermochemical conversion methods, such as pyrolysis and gasification, typically achieve higher process efficiency by rapidly transforming biomass into syngas, bio-oil, or char with yields dependent on temperature and feedstock properties. Biochemical conversion, involving enzymatic hydrolysis and fermentation, usually results in lower energy efficiency due to longer processing times but produces high yields of specific biofuels like ethanol and biogas. Optimizing parameters such as reaction temperature in thermochemical processes and enzyme concentration in biochemical methods directly influences both conversion efficiency and overall product yields.
Environmental Impact and Sustainability
Thermochemical conversion, including pyrolysis and gasification, typically emits higher greenhouse gases and requires significant energy input, impacting air quality and carbon footprint. Biochemical conversion processes like anaerobic digestion and fermentation usually offer lower emissions and generate renewable biofuels with reduced environmental harm. Sustainable bioenergy strategies favor biochemical methods due to enhanced carbon neutrality and improved soil health through byproduct utilization.
Technology Readiness and Scalability
Thermochemical conversion technologies, such as pyrolysis and gasification, exhibit higher technology readiness levels and greater scalability for large-scale bioenergy production compared to biochemical methods. Biochemical conversion, involving enzymatic hydrolysis and fermentation, often faces challenges in enzyme costs and process efficiency, limiting its widespread industrial deployment. Scaling thermochemical processes benefits from established industrial infrastructure and faster processing times, enhancing feasibility for commercial applications.
Economic Considerations and Cost Analysis
Thermochemical conversion processes, such as pyrolysis and gasification, typically require higher capital investment and operational costs due to advanced reactor technology and high energy input, but they offer faster conversion rates and greater feedstock flexibility. Biochemical conversion, notably fermentation and anaerobic digestion, generally involves lower upfront costs but longer processing times and sensitivity to feedstock impurities, impacting overall economic viability. Comprehensive cost analysis reveals thermochemical methods can achieve higher economies of scale, while biochemical routes benefit from established infrastructure and lower energy demands, influencing decision-making in biofuel production projects.
Applications in Energy and Chemical Production
Thermochemical conversion processes such as pyrolysis, gasification, and combustion are extensively used for producing biofuels, syngas, and heat energy from biomass feedstocks, supporting large-scale power generation and industrial chemical synthesis. Biochemical conversion employs microbial fermentation and enzymatic hydrolysis to convert biomass into bioethanol, biogas, and biochemicals, offering sustainable solutions for transportation fuels and renewable chemical production. Both methods enable the valorization of lignocellulosic feedstocks, but thermochemical routes are favored for solid fuel and high-energy outputs, while biochemical pathways excel in producing liquid biofuels and specialty chemicals.
Future Trends and Research Directions
Emerging research in thermochemical conversion emphasizes enhanced catalytic processes and integration with carbon capture technologies to improve efficiency and reduce greenhouse gas emissions. Biochemical conversion is advancing through genetic engineering of microbes and enzymes to optimize biomass breakdown and increase biofuel yields. Future trends include hybrid systems combining both methods for maximizing energy recovery and sustainability in bioenergy production.
Pyrolysis
Pyrolysis, a thermochemical conversion process, decomposes biomass at high temperatures in the absence of oxygen to produce bio-oil, syngas, and char, offering faster processing and higher energy density compared to biochemical conversion methods like fermentation.
Gasification
Gasification, a thermochemical conversion process, efficiently converts biomass into syngas through high-temperature partial oxidation, offering faster processing and higher energy yield compared to biochemical conversion methods like anaerobic digestion.
Hydrothermal liquefaction
Hydrothermal liquefaction, a thermochemical conversion process, efficiently transforms wet biomass into bio-crude oil by applying high temperature and pressure, offering faster and higher-yield biofuel production compared to traditional biochemical conversion methods like anaerobic digestion.
Combustion
Combustion, a thermochemical conversion process, rapidly converts biomass into heat and energy through oxidation at high temperatures, contrasting with slower biochemical methods that rely on microbial activity to break down organic material.
Anaerobic digestion
Anaerobic digestion, a biochemical conversion process, utilizes microorganisms to break down organic matter in the absence of oxygen, producing biogas and digestate, whereas thermochemical conversion relies on heat-driven processes like pyrolysis or gasification to transform biomass into fuels and chemicals.
Fermentation
Fermentation, a key biochemical conversion process, utilizes microorganisms to break down organic materials into biofuels, contrasting with thermochemical conversion that relies on heat and chemical reactions to transform biomass.
Enzymatic hydrolysis
Enzymatic hydrolysis, a key biochemical conversion process, uses specific enzymes to break down biomass into fermentable sugars, offering higher selectivity and lower energy consumption compared to thermochemical conversion methods.
Syngas production
Thermochemical conversion produces syngas through high-temperature processes like gasification and pyrolysis, whereas biochemical conversion generates syngas less efficiently, primarily focusing on fermentation and enzymatic breakdown methods.
Bio-oil synthesis
Thermochemical conversion produces bio-oil through high-temperature processes like pyrolysis and gasification, while biochemical conversion synthesizes bio-oil by enzymatic fermentation of biomass-derived sugars.
Lignocellulosic biomass valorization
Thermochemical conversion of lignocellulosic biomass efficiently produces bio-oil, syngas, and char through pyrolysis and gasification, while biochemical conversion utilizes enzymatic hydrolysis and fermentation to generate bioethanol and biochemicals from cellulose and hemicellulose fractions.
Thermochemical conversion vs Biochemical conversion Infographic
 njnir.com
njnir.com