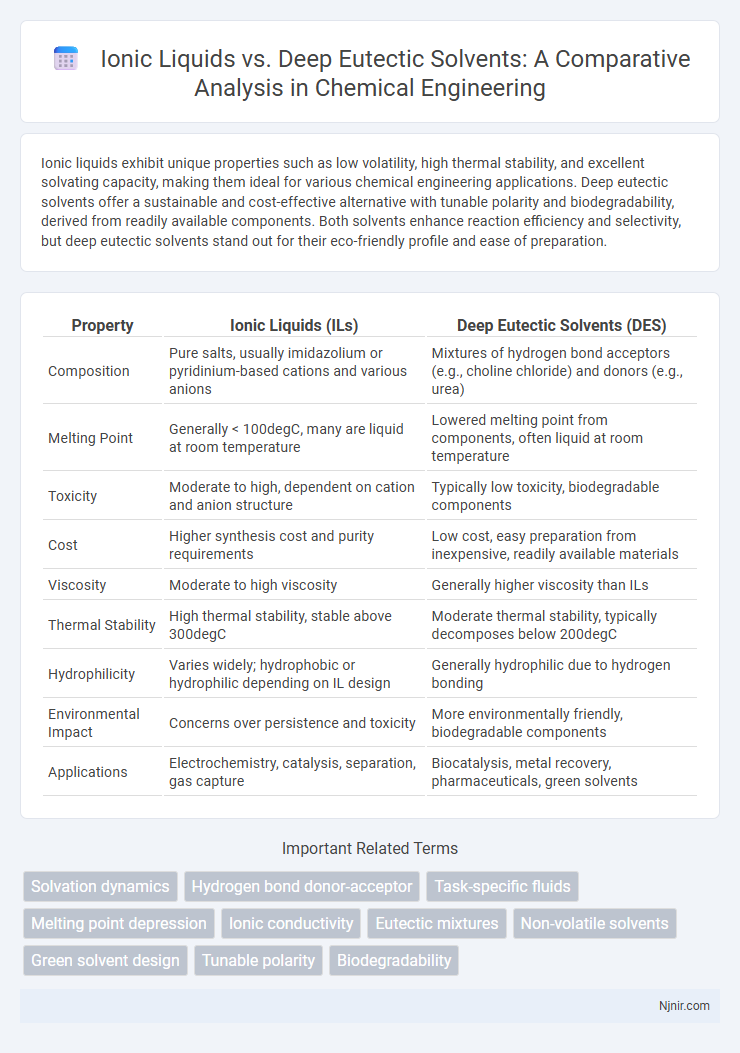
Ionic liquids exhibit unique properties such as low volatility, high thermal stability, and excellent solvating capacity, making them ideal for various chemical engineering applications. Deep eutectic solvents offer a sustainable and cost-effective alternative with tunable polarity and biodegradability, derived from readily available components. Both solvents enhance reaction efficiency and selectivity, but deep eutectic solvents stand out for their eco-friendly profile and ease of preparation.
Table of Comparison
| Property | Ionic Liquids (ILs) | Deep Eutectic Solvents (DES) |
|---|---|---|
| Composition | Pure salts, usually imidazolium or pyridinium-based cations and various anions | Mixtures of hydrogen bond acceptors (e.g., choline chloride) and donors (e.g., urea) |
| Melting Point | Generally < 100degC, many are liquid at room temperature | Lowered melting point from components, often liquid at room temperature |
| Toxicity | Moderate to high, dependent on cation and anion structure | Typically low toxicity, biodegradable components |
| Cost | Higher synthesis cost and purity requirements | Low cost, easy preparation from inexpensive, readily available materials |
| Viscosity | Moderate to high viscosity | Generally higher viscosity than ILs |
| Thermal Stability | High thermal stability, stable above 300degC | Moderate thermal stability, typically decomposes below 200degC |
| Hydrophilicity | Varies widely; hydrophobic or hydrophilic depending on IL design | Generally hydrophilic due to hydrogen bonding |
| Environmental Impact | Concerns over persistence and toxicity | More environmentally friendly, biodegradable components |
| Applications | Electrochemistry, catalysis, separation, gas capture | Biocatalysis, metal recovery, pharmaceuticals, green solvents |
Introduction: Ionic Liquids and Deep Eutectic Solvents
Ionic liquids (ILs) are salts composed of organic cations and inorganic or organic anions, known for their low volatility, high thermal stability, and tunable solvation properties. Deep eutectic solvents (DESs) consist of a eutectic mixture of hydrogen bond donors and acceptors, exhibiting biodegradability, low toxicity, and cost-effectiveness. Both ILs and DESs serve as green solvents with distinct physicochemical characteristics, making them suitable for diverse applications in catalysis, electrochemistry, and sustainable synthesis.
Structural Differences: ILs vs DES
Ionic liquids (ILs) are composed entirely of ions, typically featuring bulky, asymmetrical organic cations paired with inorganic or organic anions, leading to a low melting point and high ionic conductivity. Deep eutectic solvents (DES) form via a eutectic mixture of a hydrogen bond donor and an acceptor, resulting in a liquid phase at a temperature significantly lower than that of the individual components without fully dissociating into free ions. The fundamental structural difference lies in ILs' discrete ionic nature versus DES's extensive hydrogen-bond network, influencing their physicochemical properties and applications.
Physicochemical Properties Comparison
Ionic liquids exhibit low volatility, high thermal stability, and wide electrochemical windows, which make them suitable for various high-performance applications. Deep eutectic solvents (DESs) typically possess lower toxicity, biodegradability, and easier preparation methods, but often have higher viscosity and narrower electrochemical stability. The tunable polarity and hydrogen-bonding networks in DESs contrast with the ionic nature and charge-delocalization found in ionic liquids, influencing their solvation abilities and conductivity profiles.
Synthesis Methods and Green Chemistry
Ionic liquids are synthesized through ion exchange or quaternization reactions requiring energy-intensive processes, whereas deep eutectic solvents (DES) form via simple mixing of hydrogen bond donors and acceptors under mild conditions, offering a more sustainable approach. DES exhibit biodegradability and lower toxicity, aligning closely with green chemistry principles by minimizing hazardous reagents and waste generation. The lower synthesis cost and environmentally benign nature of DES make them preferable for applications seeking atom economy and reduced ecological impact.
Solubility and Selectivity for Chemical Engineering
Ionic liquids exhibit high solubility for a wide range of organic and inorganic compounds due to their tunable ionic structures, enabling precise selectivity in separation and catalysis processes essential for chemical engineering applications. Deep eutectic solvents, composed of hydrogen bond donors and acceptors, offer comparable solubility for polar and non-polar substances but often provide enhanced biocompatibility and lower toxicity, which improves selective extraction in environmentally sensitive processes. The choice between ionic liquids and deep eutectic solvents depends on the target compound's polarity, process temperature, and required selectivity for optimizing reaction efficiency and product purity.
Thermal and Chemical Stability
Ionic liquids exhibit superior thermal stability, often with decomposition temperatures above 300degC, making them ideal for high-temperature applications, whereas deep eutectic solvents (DES) typically have lower thermal stability, decomposing around 150-200degC. Chemically, ionic liquids are highly resistant to oxidative and reductive conditions due to their strong ionic bonds, while DES may undergo chemical degradation faster because of their hydrogen-bonded complex structures. The enhanced stability of ionic liquids enables their use in aggressive chemical environments and long-term thermal processes, contrasting with the more biodegradable and environmentally benign profile of DES.
Applications in Separation Processes
Ionic liquids exhibit exceptional selectivity and thermal stability in gas separation, solvent extraction, and membrane processes, enhancing efficiency in CO2 capture and removal of heavy metals. Deep eutectic solvents (DES) offer a cost-effective and biodegradable alternative for separating organic compounds, biomass extraction, and metal ion recovery due to their tunable polarity and low volatility. Both solvents enable greener separation technologies with reduced environmental impact, but ionic liquids are preferred for high-performance industrial applications while DES are favored in sustainable and large-scale processes.
Environmental Impact and Toxicity
Ionic liquids often exhibit low vapor pressure, reducing air pollution, but some have high toxicity and limited biodegradability, raising environmental concerns. Deep eutectic solvents tend to be biodegradable and derived from renewable components, resulting in lower toxicity and enhanced environmental compatibility. Both solvent types require thorough assessment of their life cycle impacts for sustainable industrial application.
Economic and Scalability Considerations
Ionic liquids often involve high synthesis costs and complex purification processes, limiting their economic viability and large-scale application. Deep eutectic solvents (DES) present a more cost-effective and scalable alternative due to their simple preparation from inexpensive, readily available components and lower energy requirements. The industrial adoption of DES benefits from their biodegradability and tunable properties, enhancing sustainability while reducing operational expenses compared to ionic liquids.
Future Trends and Research Directions
Future trends in ionic liquids focus on enhancing their thermal stability and recyclability for sustainable industrial applications, while deep eutectic solvents (DES) research prioritizes tunability and biodegradability to reduce environmental impact. Advances in computational modeling and machine learning accelerate the discovery of novel ionic liquids and DES with tailored properties for energy storage, catalysis, and pharmaceuticals. Integration of green chemistry principles drives the development of hybrid systems combining ionic liquids and deep eutectic solvents to optimize performance and cost-effectiveness in large-scale processes.
Solvation dynamics
Ionic liquids exhibit faster solvation dynamics and higher polarity compared to deep eutectic solvents, enhancing their efficiency in catalytic and electrochemical applications.
Hydrogen bond donor-acceptor
Ionic liquids typically consist of charged ions forming strong Coulombic interactions, whereas deep eutectic solvents rely heavily on hydrogen bond donor-acceptor interactions to achieve their unique solvent properties.
Task-specific fluids
Task-specific ionic liquids exhibit higher tunability and thermal stability compared to deep eutectic solvents, making them more effective for specialized catalytic and separation processes.
Melting point depression
Deep eutectic solvents exhibit greater melting point depression than ionic liquids due to their unique hydrogen-bonding interactions and eutectic composition.
Ionic conductivity
Ionic liquids exhibit higher ionic conductivity than deep eutectic solvents due to their lower viscosity and greater ion mobility, making them more efficient electrolytes in energy storage applications.
Eutectic mixtures
Deep eutectic solvents exhibit enhanced tunability and environmental friendliness compared to ionic liquids due to their eutectic mixtures formed by hydrogen bond donors and acceptors.
Non-volatile solvents
Ionic liquids are non-volatile solvents with low vapor pressure and high thermal stability, whereas deep eutectic solvents offer comparable low volatility combined with easier synthesis and biodegradability.
Green solvent design
Deep eutectic solvents offer a more sustainable and cost-effective alternative to ionic liquids in green solvent design due to their biodegradability, low toxicity, and simpler preparation methods.
Tunable polarity
Ionic liquids exhibit tunable polarity through varying cation-anion combinations, while deep eutectic solvents achieve tunable polarity primarily by adjusting hydrogen bond donor and acceptor components.
Biodegradability
Deep eutectic solvents generally exhibit higher biodegradability compared to ionic liquids due to their natural component-based composition and lower environmental persistence.
ionic liquids vs deep eutectic solvents Infographic
 njnir.com
njnir.com