Distillation relies on vapor-liquid equilibrium and thermal energy to separate components based on boiling points, making it energy-intensive for heat-sensitive compounds. Membrane separation uses selective permeable barriers to separate molecules without phase change, offering lower energy consumption and better suitability for temperature-sensitive mixtures. Membrane processes often provide higher selectivity and operational simplicity, but may face challenges with membrane fouling and limited separation range.
Table of Comparison
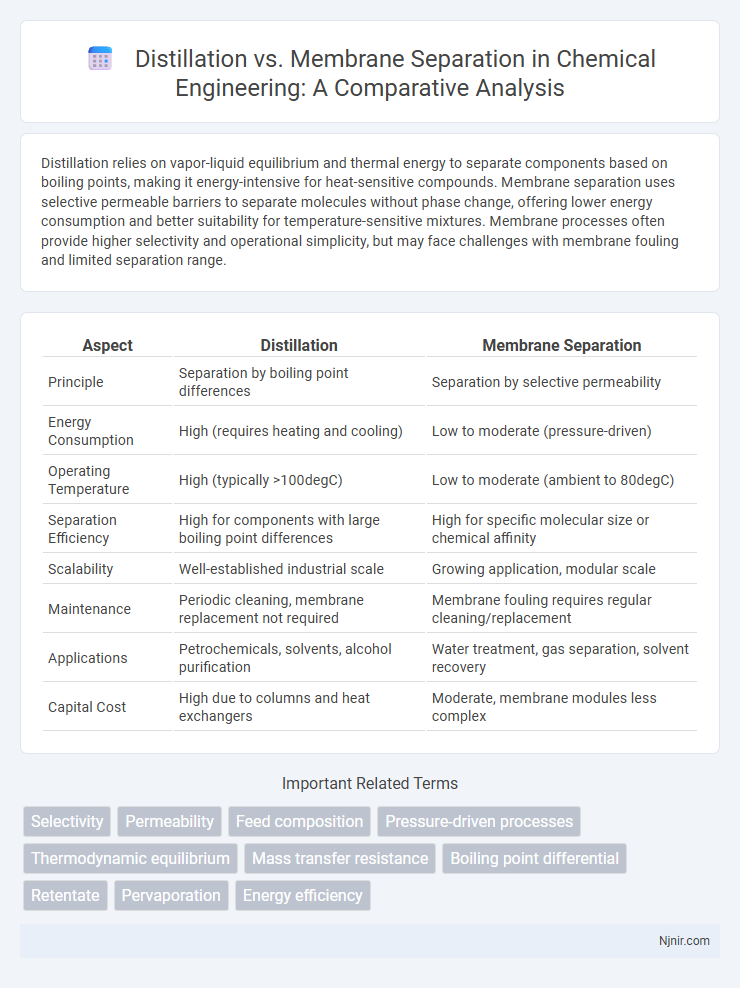
| Aspect | Distillation | Membrane Separation |
|---|---|---|
| Principle | Separation by boiling point differences | Separation by selective permeability |
| Energy Consumption | High (requires heating and cooling) | Low to moderate (pressure-driven) |
| Operating Temperature | High (typically >100degC) | Low to moderate (ambient to 80degC) |
| Separation Efficiency | High for components with large boiling point differences | High for specific molecular size or chemical affinity |
| Scalability | Well-established industrial scale | Growing application, modular scale |
| Maintenance | Periodic cleaning, membrane replacement not required | Membrane fouling requires regular cleaning/replacement |
| Applications | Petrochemicals, solvents, alcohol purification | Water treatment, gas separation, solvent recovery |
| Capital Cost | High due to columns and heat exchangers | Moderate, membrane modules less complex |
Introduction to Distillation and Membrane Separation
Distillation is a thermal separation process that relies on differences in boiling points to separate components in a liquid mixture by vaporizing and condensing them. Membrane separation uses selective permeability of membranes to separate substances based on size, charge, or chemical affinity without phase change, making it energy-efficient. Both techniques are fundamental in chemical, petroleum, and wastewater industries, offering distinct advantages depending on the mixture properties and separation requirements.
Basic Principles of Distillation
Distillation relies on the difference in boiling points of components within a liquid mixture, separating substances by vaporizing the more volatile components and then condensing the vapor into a purified liquid. This thermal process is driven by phase equilibrium and requires significant energy input for heating and cooling. Key parameters influencing distillation efficiency include relative volatility, reflux ratio, and column design, which determine the separation purity and throughput.
Fundamentals of Membrane Separation
Membrane separation relies on selective permeability, where membranes allow specific molecules or ions to pass based on size, charge, or solubility, offering energy-efficient separation compared to distillation's thermal phase change process. Key membrane types include microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, each targeting different particle sizes and molecular structures. Unlike distillation, membrane separation operates at lower temperatures and pressures, making it suitable for heat-sensitive materials and continuous processing in water treatment, food processing, and chemical industries.
Comparative Energy Efficiency
Membrane separation typically requires significantly less energy than distillation due to its reliance on pressure or concentration gradients instead of phase changes, which consume substantial heat. Distillation demands high thermal energy to vaporize and condense mixtures, making it less efficient for heat-sensitive or dilute solutions. Energy consumption in membrane processes can be reduced by optimizing membrane selectivity and permeability, leading to lower operational costs and improved sustainability in separation tasks.
Separation Efficiency and Selectivity
Distillation offers high separation efficiency for mixtures with significant volatility differences but struggles with close boiling point components due to thermodynamic limitations. Membrane separation provides superior selectivity by targeting molecular size or chemical affinity, enabling energy-efficient separation of azeotropic or heat-sensitive mixtures. Combining both methods can optimize separation processes by leveraging distillation's efficiency and membranes' selectivity for complex mixtures.
Equipment Design and Process Integration
Distillation equipment design demands tall columns, trays or packing materials, and precise temperature control to achieve effective component separation by volatility differences. Membrane separation equipment features compact modules with selective permeable membranes enabling energy-efficient, continuous separation without phase change. Process integration in distillation involves heat integration through reboilers and condensers to optimize energy usage, while membrane systems integrate seamlessly with upstream or downstream units to enhance selectivity and reduce operational costs.
Application Areas in Chemical Engineering
Distillation is widely applied in chemical engineering for separating liquid mixtures based on boiling points, crucial in petrochemical refining, solvent recovery, and alcohol purification. Membrane separation excels in processes requiring selective permeability, such as gas separation, wastewater treatment, and bioproduct purification. The choice between distillation and membrane separation depends on factors like feed composition, energy efficiency, and desired purity levels in industrial applications.
Environmental Impact and Sustainability
Distillation, a traditional separation method, is energy-intensive and generates significant greenhouse gas emissions due to high thermal energy consumption, impacting environmental sustainability negatively. Membrane separation offers a lower-energy alternative, reducing carbon footprint by operating at ambient temperatures and pressures, which enhances sustainability through decreased fossil fuel reliance. Adoption of membrane technologies aligns with global environmental goals by minimizing waste and enabling resource-efficient separation processes.
Economic Considerations and Cost Analysis
Distillation typically involves higher energy consumption due to the need for heating and vaporization, leading to increased operational costs compared to membrane separation. Membrane separation systems require lower energy input and offer reduced maintenance expenses, making them more cost-effective for large-scale applications where feed composition favors selective permeation. Capital investment for distillation units is generally higher due to complex equipment and large footprints, while membranes benefit from modular design and scalability, resulting in lower upfront costs and faster payback periods.
Future Trends and Emerging Technologies
Future trends in distillation emphasize energy efficiency improvements through intensified processes like dividing wall columns and heat integration techniques. Membrane separation advances prioritize developing high-selectivity materials such as mixed-matrix membranes and graphene-based membranes to enhance permeability and durability. Emerging technologies integrate hybrid systems combining membrane separation with distillation to optimize separation performance and reduce operational costs in industries such as petrochemical and water treatment.
Selectivity
Membrane separation offers higher selectivity through molecular size exclusion and affinity differences, while distillation relies on volatility differences, often resulting in lower selectivity for close-boiling or azeotropic mixtures.
Permeability
Membrane separation offers higher permeability compared to distillation, enabling faster mass transfer and lower energy consumption in separating mixtures.
Feed composition
Membrane separation efficiently handles feed streams with dilute components, while distillation is more effective for feed compositions with significant differences in boiling points and higher component concentrations.
Pressure-driven processes
Pressure-driven membrane separation processes like reverse osmosis and nanofiltration offer energy efficiency and selectivity advantages over traditional distillation techniques by utilizing transmembrane pressure gradients instead of thermal vaporization.
Thermodynamic equilibrium
Membrane separation operates far from thermodynamic equilibrium enabling energy-efficient separation, whereas distillation relies on vapor-liquid equilibrium requiring significant energy input for phase change.
Mass transfer resistance
Membrane separation exhibits lower mass transfer resistance compared to distillation, resulting in higher energy efficiency and enhanced separation performance.
Boiling point differential
Distillation efficiently separates mixtures with significant boiling point differentials, while membrane separation excels in separating components with similar boiling points by relying on selective permeability.
Retentate
Retentate in membrane separation contains concentrated impurities retained by the membrane, whereas distillation separates components based on boiling points, producing no retentate but rather separate liquid phases.
Pervaporation
Pervaporation, a membrane separation technique, offers energy-efficient and selective separation of liquid mixtures by combining permeation and evaporation, outperforming traditional distillation in separating azeotropes and heat-sensitive compounds.
Energy efficiency
Membrane separation consumes significantly less energy than distillation by utilizing selective permeability instead of vaporization to separate components, making it a more energy-efficient option for separation processes.
distillation vs membrane separation Infographic
 njnir.com
njnir.com