Subcritical water hydrolysis utilizes water at elevated temperatures and pressures below its critical point to break down complex organic molecules, offering an environmentally friendly alternative to traditional acid hydrolysis. This method enhances reaction rates and selectivity while minimizing the use of corrosive chemicals and hazardous waste generation. Compared to acid hydrolysis, subcritical water hydrolysis improves process safety and reduces the need for extensive neutralization and separation steps.
Table of Comparison
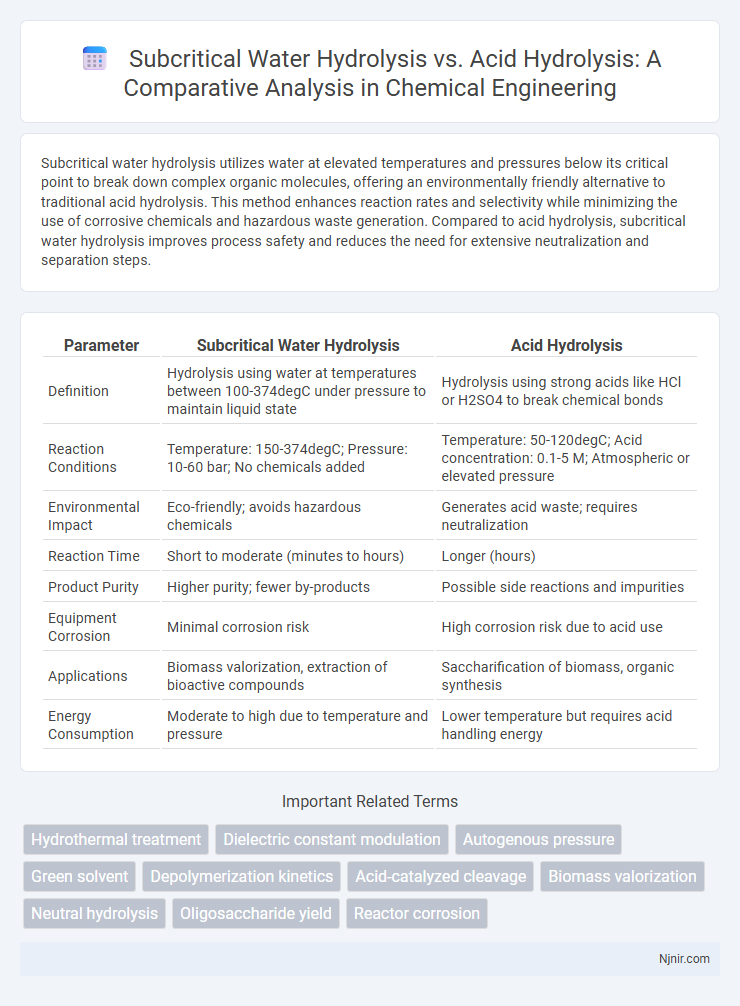
| Parameter | Subcritical Water Hydrolysis | Acid Hydrolysis |
|---|---|---|
| Definition | Hydrolysis using water at temperatures between 100-374degC under pressure to maintain liquid state | Hydrolysis using strong acids like HCl or H2SO4 to break chemical bonds |
| Reaction Conditions | Temperature: 150-374degC; Pressure: 10-60 bar; No chemicals added | Temperature: 50-120degC; Acid concentration: 0.1-5 M; Atmospheric or elevated pressure |
| Environmental Impact | Eco-friendly; avoids hazardous chemicals | Generates acid waste; requires neutralization |
| Reaction Time | Short to moderate (minutes to hours) | Longer (hours) |
| Product Purity | Higher purity; fewer by-products | Possible side reactions and impurities |
| Equipment Corrosion | Minimal corrosion risk | High corrosion risk due to acid use |
| Applications | Biomass valorization, extraction of bioactive compounds | Saccharification of biomass, organic synthesis |
| Energy Consumption | Moderate to high due to temperature and pressure | Lower temperature but requires acid handling energy |
Introduction to Hydrolysis Techniques in Chemical Engineering
Hydrolysis techniques in chemical engineering primarily involve breaking down complex molecules using water or acids as catalysts, with subcritical water hydrolysis leveraging water at temperatures between 100degC and 374degC under high pressure to enhance reaction rates without corrosive solvents. Acid hydrolysis employs strong acids like sulfuric or hydrochloric acid to cleave chemical bonds, often requiring lower temperatures but generating hazardous waste and necessitating corrosion-resistant equipment. Subcritical water hydrolysis offers an environmentally friendly alternative with tunable solvent properties and reduced waste, making it favorable for biomass conversion, whereas acid hydrolysis remains widely used for its simplicity and effectiveness in carbohydrate and protein processing.
Fundamentals of Subcritical Water Hydrolysis
Subcritical water hydrolysis operates at temperatures between 100degC and 374degC under high pressure, maintaining water in a liquid state with enhanced ionization and solvent properties, which accelerates the breakdown of complex biomolecules without added chemicals. This method leverages the unique dielectric constant and dielectric permittivity of water under subcritical conditions to selectively hydrolyze carbohydrates, proteins, and lipids into valuable monomers like sugars and amino acids. The fundamental advantage lies in its eco-friendly, catalyst-free process that increases reaction rates and efficiency compared to conventional acid hydrolysis, which relies on strong acids and often requires additional neutralization steps.
Principles of Acid Hydrolysis
Acid hydrolysis involves breaking down complex molecules by using strong acids like sulfuric or hydrochloric acid, which protonate glycosidic bonds in polysaccharides, leading to cleavage into simpler sugars. This process typically requires elevated temperatures and controlled acid concentrations to optimize yield while minimizing degradation of sensitive components. In contrast, subcritical water hydrolysis uses high-temperature water under pressure as a green solvent, offering a catalyst-free alternative that reduces hazardous waste and enhances reaction rates.
Comparative Reaction Mechanisms
Subcritical water hydrolysis operates by using high-temperature, pressurized water below its critical point to cleave chemical bonds via enhanced ionization and increased diffusivity, facilitating efficient breakdown of biomass with minimal acid catalysts. Acid hydrolysis relies on protonation of glycosidic linkages through strong mineral acids like sulfuric acid, leading to rapid hydrolysis but often accompanied by sugar degradation and formation of inhibitory byproducts. The key difference lies in subcritical water's ability to act both as a solvent and catalyst under controlled thermal conditions, enabling selective hydrolysis with reduced environmental impact compared to the corrosive and harsh conditions required in acid hydrolysis.
Efficiency and Yield of Hydrolysis Processes
Subcritical water hydrolysis demonstrates higher efficiency and yield compared to acid hydrolysis due to its ability to maintain elevated temperatures and pressures without corrosive reagents, enhancing the breakdown of complex biomass into fermentable sugars. This process offers faster reaction rates and minimizes sugar degradation, resulting in higher sugar recovery yields, often exceeding 80%. In contrast, acid hydrolysis typically suffers from lower yields due to sugar degradation and requires neutralization steps, which add to processing costs and complexity.
Environmental Impact Assessment
Subcritical water hydrolysis significantly reduces environmental impact by eliminating the need for hazardous acids and minimizing toxic waste generation compared to acid hydrolysis. This green technology operates under high temperature and pressure conditions, promoting safer and more sustainable biomass processing without producing corrosive byproducts. Life cycle assessments reveal that subcritical water hydrolysis lowers energy consumption and greenhouse gas emissions, aligning with stringent environmental regulations and sustainability goals.
Operational Safety Considerations
Subcritical water hydrolysis operates at moderate temperatures and pressures, significantly reducing the risks associated with handling strong acids in acid hydrolysis, which involves highly corrosive and hazardous chemicals. The closed systems used in subcritical water processes minimize exposure to toxic substances, enhancing operational safety by preventing chemical spills and emissions. In contrast, acid hydrolysis requires stringent safety protocols to manage acid storage, disposal, and neutralization, increasing the complexity and potential hazards of the process.
Process Optimization and Scalability
Subcritical water hydrolysis offers enhanced process optimization by operating at moderate temperatures and pressures, which reduces chemical waste and improves reaction rates compared to traditional acid hydrolysis. Acid hydrolysis requires strong acids, posing challenges in corrosion management and extensive neutralization steps, limiting its scalability in industrial applications. The scalability of subcritical water hydrolysis benefits from simpler reactor designs and lower environmental impact, facilitating continuous processing and easier integration into biorefinery systems.
Substrate Versatility and Product Purity
Subcritical water hydrolysis offers greater substrate versatility by effectively processing a wide range of biomass materials, including lignocellulosic feedstocks, without requiring harsh chemical additives. This method enhances product purity by minimizing the formation of undesirable by-products and avoiding metal ion contamination often associated with acid hydrolysis. In contrast, acid hydrolysis can degrade sensitive substrates and typically requires extensive neutralization steps, leading to lower overall product purity and higher environmental concerns.
Industrial Applications and Future Perspectives
Subcritical water hydrolysis offers a greener and more sustainable alternative to acid hydrolysis in industrial applications, enabling efficient biomass conversion without the use of harsh chemicals or generating hazardous waste. This technology is increasingly adopted in biofuel production, pharmaceutical extraction, and food processing due to its selectivity, faster reaction rates, and lower environmental impact. Future perspectives emphasize scaling up subcritical water hydrolysis reactors, improving process integration with renewable energy sources, and enhancing catalyst development to expand its industrial viability and economic competitiveness.
Hydrothermal treatment
Subcritical water hydrolysis enhances hydrothermal treatment efficiency by using high-temperature, high-pressure water to rapidly break down biomass without corrosive chemicals, unlike acid hydrolysis which relies on strong acids and poses higher environmental and safety risks.
Dielectric constant modulation
Subcritical water hydrolysis enhances biomass conversion by lowering water's dielectric constant to around 25-30, mimicking organic solvents and facilitating hydrolysis, whereas acid hydrolysis relies on low pH but maintains a high dielectric constant near 80, affecting reaction specificity and efficiency.
Autogenous pressure
Subcritical water hydrolysis operates under autogenous pressure generated by heating water below its critical point, whereas acid hydrolysis requires externally applied pressure or atmospheric conditions without relying on autogenous pressure.
Green solvent
Subcritical water hydrolysis utilizes environmentally friendly water under high temperature and pressure as a green solvent, reducing toxic acid use and minimizing hazardous waste compared to traditional acid hydrolysis.
Depolymerization kinetics
Subcritical water hydrolysis accelerates depolymerization kinetics by enhancing molecular cleavage through elevated temperature and pressure, while acid hydrolysis relies on proton catalysis to break polymer chains, typically resulting in slower reaction rates and increased catalyst corrosion.
Acid-catalyzed cleavage
Acid-catalyzed cleavage in acid hydrolysis efficiently breaks down complex biomolecules into simpler monomers by protonating glycosidic bonds, whereas subcritical water hydrolysis leverages high-temperature water under pressure to achieve cleavage without strong acids.
Biomass valorization
Subcritical water hydrolysis enhances biomass valorization by efficiently breaking down lignocellulosic materials into valuable biofuels and biochemicals without corrosive acids, offering a greener and more sustainable alternative to traditional acid hydrolysis.
Neutral hydrolysis
Subcritical water hydrolysis offers a neutral hydrolysis environment that avoids the use of strong acids, enabling efficient biomass conversion with reduced corrosion and environmental impact compared to acid hydrolysis.
Oligosaccharide yield
Subcritical water hydrolysis produces higher oligosaccharide yields than acid hydrolysis due to its enhanced efficiency in breaking down polysaccharides without excessive degradation.
Reactor corrosion
Subcritical water hydrolysis significantly reduces reactor corrosion compared to acid hydrolysis due to the absence of strong corrosive acids and milder operating conditions.
Subcritical water hydrolysis vs Acid hydrolysis Infographic
 njnir.com
njnir.com