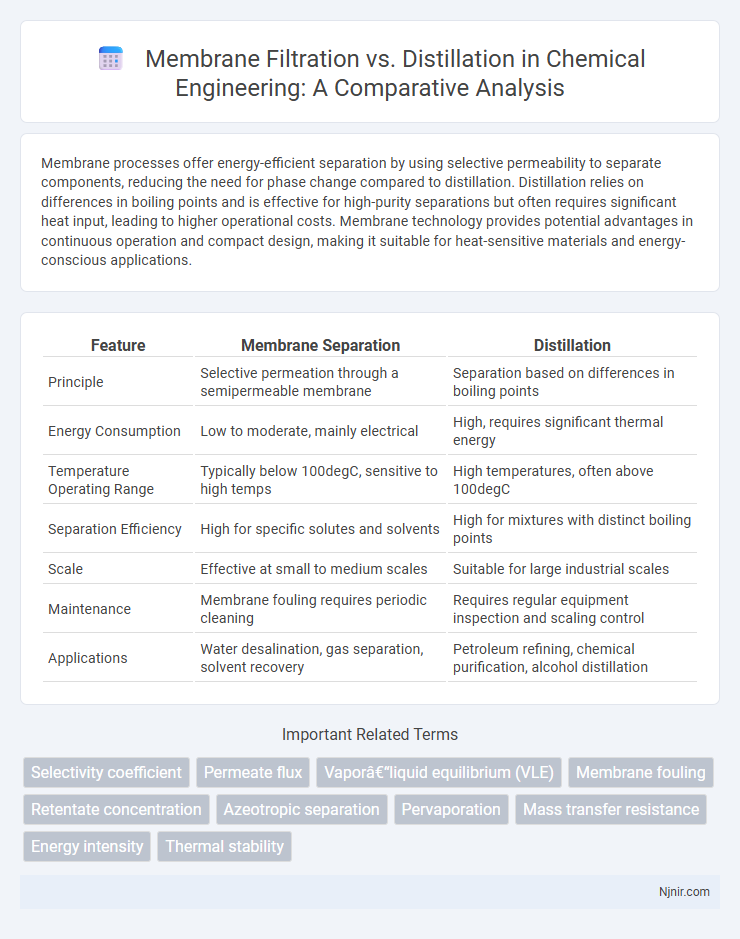
Membrane processes offer energy-efficient separation by using selective permeability to separate components, reducing the need for phase change compared to distillation. Distillation relies on differences in boiling points and is effective for high-purity separations but often requires significant heat input, leading to higher operational costs. Membrane technology provides potential advantages in continuous operation and compact design, making it suitable for heat-sensitive materials and energy-conscious applications.
Table of Comparison
| Feature | Membrane Separation | Distillation |
|---|---|---|
| Principle | Selective permeation through a semipermeable membrane | Separation based on differences in boiling points |
| Energy Consumption | Low to moderate, mainly electrical | High, requires significant thermal energy |
| Temperature Operating Range | Typically below 100degC, sensitive to high temps | High temperatures, often above 100degC |
| Separation Efficiency | High for specific solutes and solvents | High for mixtures with distinct boiling points |
| Scale | Effective at small to medium scales | Suitable for large industrial scales |
| Maintenance | Membrane fouling requires periodic cleaning | Requires regular equipment inspection and scaling control |
| Applications | Water desalination, gas separation, solvent recovery | Petroleum refining, chemical purification, alcohol distillation |
Introduction to Membrane and Distillation Processes
Membrane processes utilize semi-permeable barriers to selectively separate components based on size or chemical affinity, enabling efficient filtration and purification in industries such as water treatment and biotechnology. Distillation relies on thermal energy to separate mixtures by exploiting differences in boiling points, making it a fundamental technique in chemical production and petroleum refining. Both methods offer distinct advantages depending on the application, with membranes providing energy-efficient separation at lower temperatures and distillation enabling precise separation of complex liquid mixtures.
Fundamental Principles of Membrane Separation
Membrane separation relies on selective permeability, where a semipermeable membrane allows certain molecules or ions to pass while blocking others based on size, charge, or chemical affinity, enabling efficient separation processes. The driving forces for membrane separation include pressure, concentration gradient, or electrical potential, facilitating mechanisms such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. This method contrasts with distillation, which depends on phase changes and differences in volatility, making membrane processes more energy-efficient for separating heat-sensitive or similar boiling point mixtures.
Core Mechanisms of Distillation Techniques
Distillation techniques rely on the core mechanism of phase separation through boiling and condensation to separate mixtures based on differences in volatility. This process exploits the variation in boiling points, where the more volatile component vaporizes first and is collected after condensation, enhancing purity. Compared to membrane separation, distillation is driven by thermal energy and phase change, making it effective for separating liquid mixtures with significant boiling point differences.
Comparative Energy Efficiency
Membrane processes such as reverse osmosis typically consume 3-5 kWh per cubic meter of water, making them more energy-efficient compared to thermal distillation methods like multi-stage flash, which can require 25-40 kWh per cubic meter. Membrane technology operates at ambient temperatures and relies on pressure-driven separation, reducing overall energy demands. Distillation involves phase changes that require substantial heat input, resulting in significantly higher operational energy consumption and costs.
Selectivity and Separation Performance
Membrane separation relies on selective permeability of materials, enabling high specificity in separating molecules based on size or chemical affinity, often achieving precise separation performance in applications like gas and liquid separations. In contrast, distillation separates components based on differences in boiling points, offering robust separation for mixtures with significant volatility differences but typically lower selectivity for close-boiling or azeotropic mixtures. Membrane processes provide higher selectivity and energy efficiency in separating components with subtle chemical or physical property differences, while distillation excels in large-scale applications requiring phase change.
Cost Analysis: CAPEX and OPEX Considerations
Membrane systems generally require lower CAPEX due to simpler installation and modular scalability, while distillation units typically incur higher initial costs from complex equipment and energy-intensive infrastructure. In OPEX terms, membranes offer reduced operational expenses through lower energy consumption and easier maintenance, whereas distillation processes demand significant ongoing costs driven by high thermal energy use and frequent material replacement. Evaluating cost efficiency involves balancing membrane longevity and throughput against distillation's robustness and ability to handle diverse feedstocks.
Environmental Impact and Sustainability
Membrane separation technologies consume less energy and produce lower greenhouse gas emissions compared to distillation, enhancing environmental sustainability in industrial applications. Distillation often requires high thermal energy input, leading to increased carbon footprint and greater environmental strain, especially in large-scale processes. Implementing membrane systems reduces water and chemical usage while enabling energy-efficient separation, aligning with sustainable production goals.
Industrial Applications and Case Studies
Membrane technology, including reverse osmosis and nanofiltration, is extensively used in industrial wastewater treatment and desalination due to its energy efficiency and ability to remove contaminants with high selectivity. Distillation remains prevalent in chemical manufacturing and petrochemical industries where separation of complex mixtures and recovery of solvents is critical, despite its higher energy consumption. Case studies highlight membrane systems reducing operational costs by 30% in textile effluent treatment, while distillation units demonstrate superior purity levels exceeding 99.5% in pharmaceutical ingredient recovery.
Limitations and Challenges of Each Process
Membrane processes face limitations such as membrane fouling, limited chemical resistance, and flux decline over time, which reduce efficiency and increase operational costs. Distillation encounters challenges including high energy consumption, sensitivity to feed composition variations, and difficulties managing azeotropes or close boiling point mixtures. Both methods require careful consideration of feed characteristics and environmental factors to optimize performance and ensure cost-effectiveness.
Future Trends in Separation Technologies
Future trends in separation technologies emphasize the integration of membrane processes with distillation to enhance energy efficiency and selectivity. Advanced materials like graphene-based membranes and hybrid systems combining pervaporation with membrane distillation are being developed to reduce operational costs and environmental impact. Innovations in process intensification and smart control systems are expected to drive the next generation of sustainable separation solutions.
Selectivity coefficient
Membrane processes typically exhibit higher selectivity coefficients than distillation for separating close-boiling or azeotropic mixtures, enabling more energy-efficient and precise molecular separations.
Permeate flux
Membrane processes typically achieve higher permeate flux rates than distillation due to lower energy requirements and enhanced selectivity in separating components.
Vapor–liquid equilibrium (VLE)
Vapor-liquid equilibrium (VLE) determines the efficiency of membrane separation and distillation by influencing phase compositions and driving forces for mass transfer.
Membrane fouling
Membrane fouling, caused by particle accumulation and biofilm formation, significantly reduces filtration efficiency and lifespan in membrane-based separation compared to the lower fouling susceptibility in thermal distillation processes.
Retentate concentration
Membrane processes typically achieve higher retentate concentration by selectively filtering solutes, whereas distillation relies on phase change with less precise concentration control in the retentate.
Azeotropic separation
Membrane separation offers energy-efficient Azeotropic separation by selectively permeating specific components, while distillation relies on vapor-liquid equilibrium but often requires azeotrope-breaking agents or pressure-swing techniques.
Pervaporation
Pervaporation membranes selectively separate liquid mixtures by partial vaporization through a dense polymer or ceramic layer, offering energy-efficient alternative to traditional distillation for azeotropic or heat-sensitive mixtures.
Mass transfer resistance
Membrane processes exhibit lower mass transfer resistance compared to distillation, resulting in higher energy efficiency and faster separation rates for liquid mixtures.
Energy intensity
Membrane separation technologies typically exhibit lower energy intensity compared to thermal distillation methods due to their reliance on pressure-driven processes rather than heat input.
Thermal stability
Membrane processes typically exhibit higher thermal stability compared to distillation methods, enabling efficient separation at elevated temperatures without material degradation.
Membrane vs Distillation Infographic
 njnir.com
njnir.com