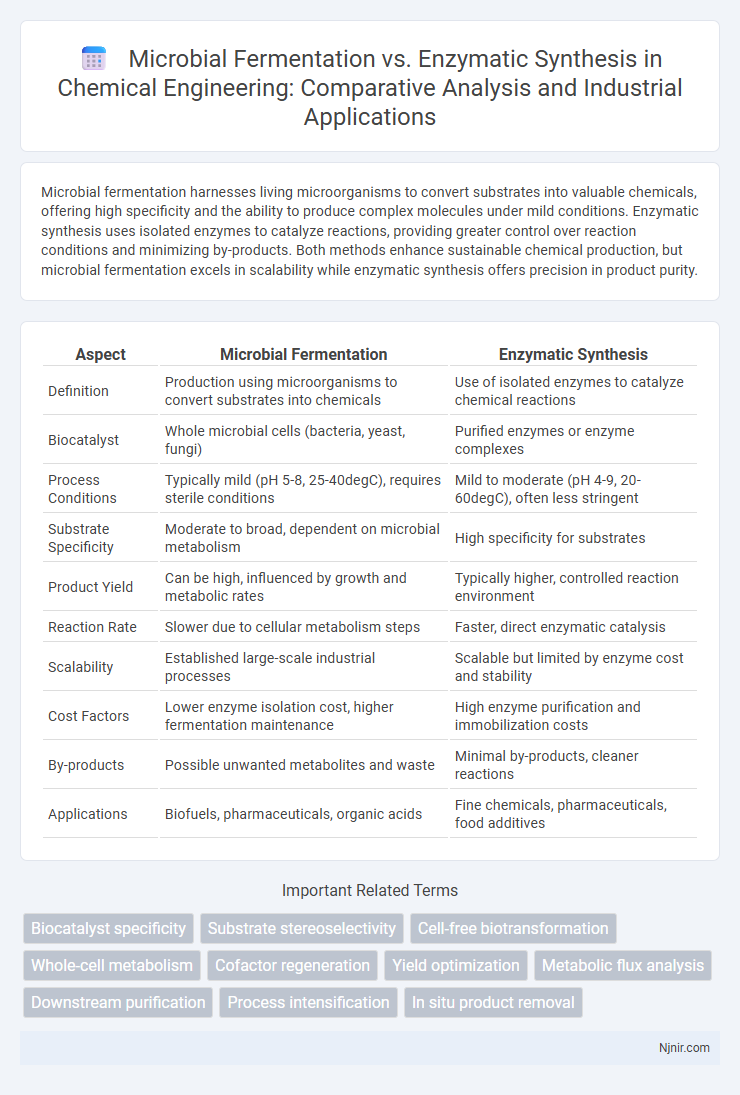
Microbial fermentation harnesses living microorganisms to convert substrates into valuable chemicals, offering high specificity and the ability to produce complex molecules under mild conditions. Enzymatic synthesis uses isolated enzymes to catalyze reactions, providing greater control over reaction conditions and minimizing by-products. Both methods enhance sustainable chemical production, but microbial fermentation excels in scalability while enzymatic synthesis offers precision in product purity.
Table of Comparison
| Aspect | Microbial Fermentation | Enzymatic Synthesis |
|---|---|---|
| Definition | Production using microorganisms to convert substrates into chemicals | Use of isolated enzymes to catalyze chemical reactions |
| Biocatalyst | Whole microbial cells (bacteria, yeast, fungi) | Purified enzymes or enzyme complexes |
| Process Conditions | Typically mild (pH 5-8, 25-40degC), requires sterile conditions | Mild to moderate (pH 4-9, 20-60degC), often less stringent |
| Substrate Specificity | Moderate to broad, dependent on microbial metabolism | High specificity for substrates |
| Product Yield | Can be high, influenced by growth and metabolic rates | Typically higher, controlled reaction environment |
| Reaction Rate | Slower due to cellular metabolism steps | Faster, direct enzymatic catalysis |
| Scalability | Established large-scale industrial processes | Scalable but limited by enzyme cost and stability |
| Cost Factors | Lower enzyme isolation cost, higher fermentation maintenance | High enzyme purification and immobilization costs |
| By-products | Possible unwanted metabolites and waste | Minimal by-products, cleaner reactions |
| Applications | Biofuels, pharmaceuticals, organic acids | Fine chemicals, pharmaceuticals, food additives |
Introduction to Microbial Fermentation and Enzymatic Synthesis
Microbial fermentation harnesses the metabolic processes of microorganisms such as bacteria, yeast, and fungi to convert substrates into valuable products like alcohol, organic acids, and antibiotics under anaerobic or aerobic conditions. Enzymatic synthesis utilizes isolated enzymes as biocatalysts to facilitate specific biochemical reactions with high selectivity and efficiency, often producing complex molecules under mild conditions. Both methodologies play crucial roles in biotechnology, with microbial fermentation emphasizing whole-cell systems and enzymatic synthesis focusing on enzyme-catalyzed transformations.
Fundamental Principles and Mechanisms
Microbial fermentation relies on living microorganisms such as bacteria, yeast, or fungi to convert substrates into desired products through complex metabolic pathways involving enzymes naturally present in the cells. Enzymatic synthesis uses isolated or immobilized enzymes to catalyze specific biochemical reactions under controlled conditions without the need for living cells, enabling higher specificity and faster reaction rates. Both processes depend on enzyme activity but differ fundamentally in scalability, control over reaction conditions, and product diversity due to the complexity of cellular metabolism in fermentation.
Raw Materials and Substrate Utilization
Microbial fermentation utilizes renewable raw materials such as sugars, starches, and agricultural biomass, efficiently converting diverse substrates into target products through biological metabolic pathways. Enzymatic synthesis relies on purified enzymes that act on specific substrates, often requiring high-purity raw materials like chemically defined compounds or synthetically derived intermediates for optimized reaction specificity. Substrate utilization in microbial fermentation is broad and adaptable, while enzymatic synthesis offers precise transformation with controlled substrate specificity.
Process Conditions and Operational Parameters
Microbial fermentation operates under controlled temperature ranges typically between 30degC to 40degC, neutral pH levels, and anaerobic or aerobic conditions depending on the microorganism used, with parameters such as agitation speed, oxygen transfer rate, and substrate concentration critically influencing product yield. Enzymatic synthesis requires precise control of specific pH values, often between 4 and 8, optimum temperature usually around 30degC to 60degC, and substrate specificity under mild conditions to maintain enzyme activity and stability, while parameters like enzyme concentration, reaction time, and cofactor availability are essential for efficiency. Both processes demand stringent monitoring of environmental factors to optimize product formation, but microbial fermentation involves living cells with complex metabolic regulation, whereas enzymatic synthesis relies on isolated biocatalysts with more defined operational parameters.
Product Yield and Specificity
Microbial fermentation typically achieves higher product yield by utilizing whole-cell systems that efficiently convert substrates into desired metabolites, often in large-scale bioreactors. Enzymatic synthesis offers superior specificity by employing isolated enzymes that catalyze targeted reactions with minimal byproducts, enhancing purity and reducing downstream processing. Balancing yield and specificity depends on process optimization, substrate selection, and enzyme engineering to maximize overall productivity in biomanufacturing.
Advantages and Limitations of Microbial Fermentation
Microbial fermentation offers high specificity and cost-effective production of complex organic compounds, leveraging natural microbial metabolism to generate diverse bio-products such as antibiotics, enzymes, and biofuels. Its advantages include the ability to scale up using renewable resources and the potential for genetic manipulation to enhance yields and product profiles. Limitations involve slower reaction rates compared to enzymatic synthesis, susceptibility to contamination, and challenges in maintaining optimal fermentation conditions for consistent quality and productivity.
Advantages and Limitations of Enzymatic Synthesis
Enzymatic synthesis offers high specificity and mild reaction conditions, resulting in fewer byproducts and greater product purity compared to microbial fermentation. However, limitations include enzyme instability under industrial conditions, high production costs, and the need for precise control of reaction parameters to maintain enzyme activity. Despite these challenges, enzymatic synthesis enables the production of complex molecules not easily achievable by microbial fermentation.
Environmental and Economic Considerations
Microbial fermentation offers sustainable production by utilizing renewable biomass, reducing reliance on fossil fuels, and generating fewer greenhouse gas emissions compared to enzymatic synthesis. Enzymatic synthesis, while often faster and producing fewer byproducts, can involve expensive enzyme catalysts and energy-intensive processes that raise overall costs and environmental impact. Evaluating lifecycle assessments reveals microbial fermentation's advantages in reducing carbon footprint and enhancing cost-efficiency for large-scale biomanufacturing applications.
Industrial Applications and Case Studies
Microbial fermentation harnesses microorganisms like bacteria and fungi to produce biofuels, pharmaceuticals, and food additives, offering cost-effective scalability and complex molecule synthesis. Enzymatic synthesis utilizes isolated enzymes for precise biocatalysis in producing fine chemicals, flavor compounds, and pharmaceuticals, enabling high specificity and mild reaction conditions. Industrial case studies highlight microbial fermentation's role in antibiotic manufacturing by companies like Pfizer, while enzymatic synthesis is pivotal in producing chiral intermediates for drugs at firms such as Novozymes.
Future Trends and Technological Innovations
Microbial fermentation is evolving with advancements in metabolic engineering and synthetic biology, enabling the production of complex bio-based compounds at higher yields and reduced costs. Enzymatic synthesis benefits from protein engineering and immobilization techniques that enhance enzyme stability and specificity, driving more sustainable and efficient industrial processes. Future trends emphasize integrating machine learning with high-throughput screening to accelerate the discovery of novel enzymes and optimize fermentation pathways for diverse biotechnological applications.
Biocatalyst specificity
Microbial fermentation utilizes living microorganisms as biocatalysts with high substrate specificity, while enzymatic synthesis employs isolated enzymes offering precise catalytic activity tailored to specific biochemical reactions.
Substrate stereoselectivity
Microbial fermentation exhibits high substrate stereoselectivity due to enzyme-specific metabolic pathways, whereas enzymatic synthesis allows precise control over stereoselective reactions by utilizing isolated, stereospecific enzymes.
Cell-free biotransformation
Cell-free biotransformation leverages isolated enzymes from microbial fermentation to enable controlled, efficient enzymatic synthesis of complex molecules without living cells.
Whole-cell metabolism
Whole-cell metabolism in microbial fermentation enables complex biochemical transformations through intrinsic enzymatic pathways, contrasting with enzymatic synthesis that relies on isolated enzymes for specific reactions.
Cofactor regeneration
Microbial fermentation leverages cellular metabolism for continuous cofactor regeneration, enhancing sustainability, while enzymatic synthesis often requires engineered cofactor recycling systems to maintain catalytic efficiency.
Yield optimization
Microbial fermentation achieves higher yield optimization by leveraging genetically engineered strains and controlled bioreactor conditions, whereas enzymatic synthesis offers precise substrate specificity with moderate yield improvements under optimized enzyme concentration and reaction parameters.
Metabolic flux analysis
Metabolic flux analysis quantifies intracellular reaction rates in microbial fermentation and enzymatic synthesis to optimize production pathways and enhance yield efficiency.
Downstream purification
Downstream purification of microbial fermentation typically involves complex removal of cells, proteins, and metabolites, whereas enzymatic synthesis often requires simpler purification steps due to fewer impurities in the reaction mixture.
Process intensification
Microbial fermentation achieves process intensification through high cell density cultures and metabolic engineering, while enzymatic synthesis enhances efficiency using immobilized enzymes and continuous flow reactors.
In situ product removal
In situ product removal enhances microbial fermentation by continuously extracting target compounds, reducing feedback inhibition and increasing yield, whereas enzymatic synthesis benefits less due to its typically simpler reaction environment and lower metabolite accumulation.
Microbial fermentation vs Enzymatic synthesis Infographic
 njnir.com
njnir.com