Hydrothermal synthesis enables precise control over crystal size and morphology by conducting reactions in aqueous solutions at elevated temperatures and pressures, promoting the formation of highly crystalline materials. Sol-gel synthesis involves transitioning a solution into a solid gel phase through hydrolysis and condensation of precursors, allowing for uniform particle distribution and low-temperature processing. Both methods offer unique advantages in tailoring material properties, with hydrothermal synthesis favoring crystallinity and sol-gel providing versatility in chemical composition and microstructure control.
Table of Comparison
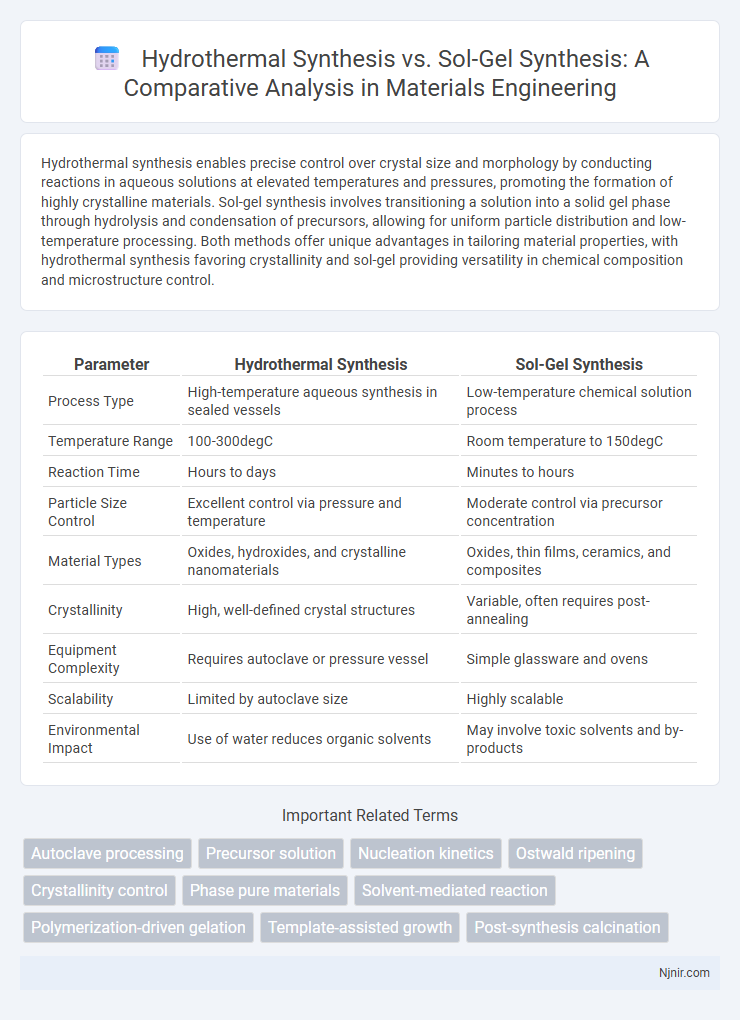
| Parameter | Hydrothermal Synthesis | Sol-Gel Synthesis |
|---|---|---|
| Process Type | High-temperature aqueous synthesis in sealed vessels | Low-temperature chemical solution process |
| Temperature Range | 100-300degC | Room temperature to 150degC |
| Reaction Time | Hours to days | Minutes to hours |
| Particle Size Control | Excellent control via pressure and temperature | Moderate control via precursor concentration |
| Material Types | Oxides, hydroxides, and crystalline nanomaterials | Oxides, thin films, ceramics, and composites |
| Crystallinity | High, well-defined crystal structures | Variable, often requires post-annealing |
| Equipment Complexity | Requires autoclave or pressure vessel | Simple glassware and ovens |
| Scalability | Limited by autoclave size | Highly scalable |
| Environmental Impact | Use of water reduces organic solvents | May involve toxic solvents and by-products |
Introduction to Hydrothermal and Sol-Gel Synthesis
Hydrothermal synthesis involves crystallizing substances from high-temperature aqueous solutions under pressure, enabling the formation of unique nanomaterials with controlled morphology and phase purity. Sol-gel synthesis relies on the transition of a system from a liquid "sol" into a solid "gel" phase, allowing precise control over the composition and microstructure of metal oxides at relatively low temperatures. Both methods are essential in materials science for producing advanced ceramics, catalysts, and nanostructured materials with tailored properties.
Fundamental Principles of Hydrothermal Synthesis
Hydrothermal synthesis relies on crystallizing substances from high-temperature aqueous solutions under high vapor pressure, promoting nucleation and crystal growth in a sealed, high-pressure environment. This method enables precise control over particle size, morphology, and phase stability by manipulating temperature, pressure, and solution chemistry. It is especially effective for producing crystalline materials like zeolites, ceramics, and nanomaterials with enhanced purity and structural integrity compared to sol-gel synthesis.
Core Concepts of Sol-Gel Synthesis
Sol-gel synthesis involves the transition of a system from a liquid "sol" into a solid "gel" phase through hydrolysis and polycondensation reactions of metal alkoxides or inorganic salts. This method allows precise control over the material's chemical composition, homogeneity, and microstructure at the molecular level, producing highly pure and uniform oxide networks. Unlike hydrothermal synthesis, sol-gel processing operates under mild conditions and is particularly advantageous for fabricating thin films, powders, and porous materials with tailored properties.
Comparative Mechanisms: Hydrothermal vs Sol-Gel Methods
Hydrothermal synthesis involves crystallizing materials from aqueous solutions at high temperature and pressure, promoting controlled nucleation and crystal growth, whereas sol-gel synthesis relies on the hydrolysis and polycondensation of metal alkoxides to form a gel network that is subsequently dried and calcined. Hydrothermal methods enable better control over crystal size, phase purity, and morphology through temperature and pressure variations, while sol-gel offers molecular-level mixing and homogeneity, which facilitates the formation of complex oxide materials with high surface area. The distinct mechanisms affect the material properties, with hydrothermal producing well-crystallized nanoparticles and sol-gel enabling versatile shapes and porous structures critical for catalysis and sensors.
Material Properties Achieved by Each Technique
Hydrothermal synthesis produces highly crystalline materials with controlled morphologies and enhanced thermal stability, often yielding uniform particle size distribution and improved phase purity. Sol-gel synthesis allows precise control over chemical composition and results in materials with high homogeneity, porosity, and tunable surface area, making it ideal for producing thin films and coatings. Both techniques enable tailoring material properties, but hydrothermal methods excel in crystallinity and phase control, while sol-gel methods optimize microstructural features and surface characteristics.
Advantages and Limitations of Hydrothermal Synthesis
Hydrothermal synthesis offers advantages such as high crystallinity, controlled particle size, and the ability to synthesize materials that are difficult to produce at ambient conditions due to its high-temperature and high-pressure aqueous environment. This method enables the growth of well-defined nanostructures with superior phase purity and fewer defects compared to sol-gel synthesis. However, limitations include the requirement for specialized high-pressure equipment, longer reaction times, and challenges in scaling up the process for industrial applications.
Pros and Cons of Sol-Gel Synthesis
Sol-gel synthesis offers advantages such as low processing temperatures, excellent control over chemical composition, and the ability to produce homogeneous and porous materials with high surface area. However, it has drawbacks including long processing times, potential shrinkage and cracking during drying, and sensitivity to environmental conditions like humidity. Compared to hydrothermal synthesis, sol-gel methods provide superior compositional control but may suffer from structural defects and less robust crystallinity.
Applications in Advanced Materials Engineering
Hydrothermal synthesis enables the growth of high-purity crystalline nanomaterials for applications in catalysts, sensors, and battery electrodes due to its ability to control particle size and morphology under high temperature and pressure. Sol-gel synthesis offers precise compositional control and homogeneity, making it ideal for fabricating advanced ceramics, thin films, and optical coatings in photonics and electronics. Both methods contribute significantly to the development of functional materials with tailored properties for aerospace, energy storage, and biomedical engineering.
Environmental and Economic Considerations
Hydrothermal synthesis often requires high pressure and temperature conditions, leading to increased energy consumption and operational costs, which can impact both economic feasibility and environmental sustainability. Sol-gel synthesis typically operates at lower temperatures and ambient pressures, reducing energy use and associated emissions, making it a more eco-friendly and cost-effective option for large-scale production. Waste management is also simpler in sol-gel processes due to fewer hazardous byproducts, whereas hydrothermal methods may require specialized handling of effluents to mitigate environmental impact.
Future Trends in Hydrothermal and Sol-Gel Synthesis
Future trends in hydrothermal and sol-gel synthesis emphasize enhanced control over nanostructure morphology and material phase purity, driven by advances in in situ monitoring and machine learning optimization. Integration of green chemistry principles is promoting the development of eco-friendly precursors and lower energy consumption processes in both methods. Emerging hybrid techniques combining hydrothermal and sol-gel approaches aim to achieve multifunctional materials with superior catalytic, optical, and electronic properties for applications in energy storage and environmental remediation.
Autoclave processing
Hydrothermal synthesis utilizes autoclave processing to enable high-pressure, high-temperature reactions in sealed vessels, promoting crystal growth and phase purity, whereas sol-gel synthesis typically occurs at lower temperatures without autoclave use, focusing on forming homogeneous gels that convert to materials through drying and calcination.
Precursor solution
Hydrothermal synthesis uses high-temperature aqueous precursor solutions in sealed reactors for crystal growth, while sol-gel synthesis relies on metal alkoxide or salt precursor solutions undergoing hydrolysis and polycondensation at lower temperatures to form gels.
Nucleation kinetics
Hydrothermal synthesis exhibits faster nucleation kinetics compared to sol-gel synthesis due to elevated temperature and pressure conditions that enhance precursor reactivity and crystal growth rates.
Ostwald ripening
Hydrothermal synthesis accelerates Ostwald ripening through high temperature and pressure, promoting larger, well-crystallized particles, whereas sol-gel synthesis involves slower Ostwald ripening under ambient conditions, resulting in smaller, more uniform nanoparticles.
Crystallinity control
Hydrothermal synthesis offers superior crystallinity control by enabling precise temperature and pressure regulation, whereas sol-gel synthesis typically produces amorphous or less crystalline materials due to lower processing temperatures.
Phase pure materials
Hydrothermal synthesis consistently produces phase-pure materials by enabling controlled temperature and pressure conditions that promote crystallinity, while sol-gel synthesis often requires post-processing annealing to achieve comparable phase purity.
Solvent-mediated reaction
Hydrothermal synthesis relies on high-temperature aqueous solvents under pressure to induce crystallization, while sol-gel synthesis employs solvent-mediated hydrolysis and polycondensation of precursors at low temperatures to form colloidal suspensions that evolve into gels.
Polymerization-driven gelation
Polymerization-driven gelation in hydrothermal synthesis typically yields more crystalline and thermally stable gels compared to the amorphous and tunable porosity gels produced via sol-gel synthesis.
Template-assisted growth
Template-assisted growth in hydrothermal synthesis yields highly crystalline nanostructures with controlled morphologies by utilizing high-temperature aqueous solutions, while sol-gel synthesis offers versatile chemical composition control and uniform gel networks at lower temperatures but often requires additional templating agents for precise structural direction.
Post-synthesis calcination
Post-synthesis calcination in hydrothermal synthesis typically enhances crystallinity and removes residual solvents at lower temperatures compared to sol-gel synthesis, which often requires higher calcination temperatures to achieve phase purity and eliminate organic precursors.
hydrothermal synthesis vs sol-gel synthesis Infographic
 njnir.com
njnir.com