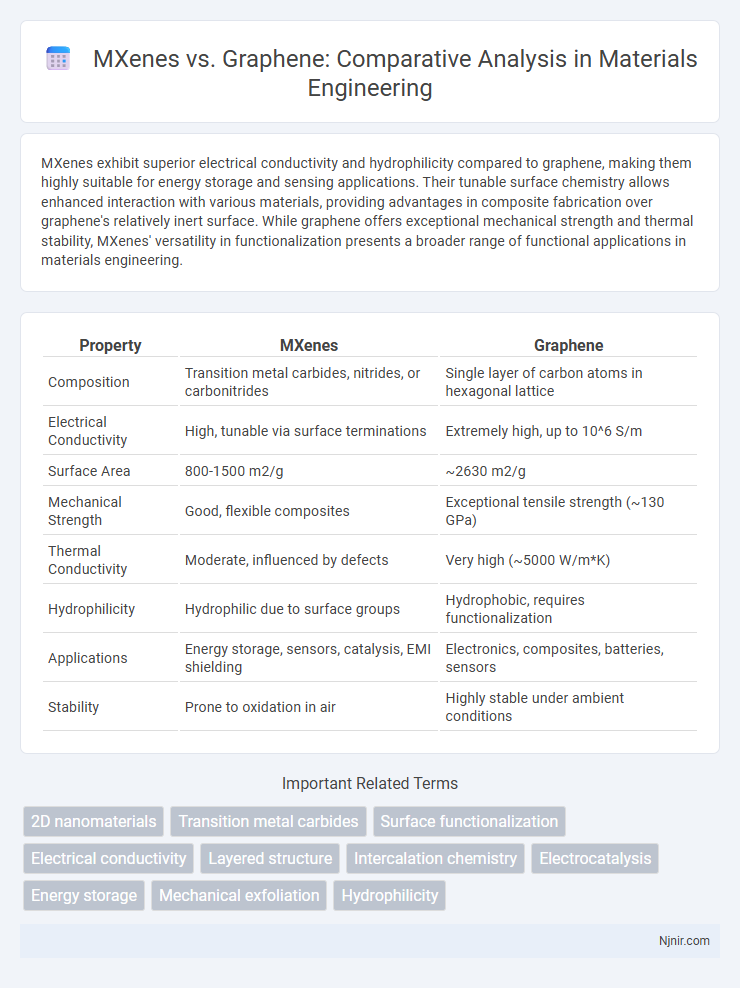
MXenes exhibit superior electrical conductivity and hydrophilicity compared to graphene, making them highly suitable for energy storage and sensing applications. Their tunable surface chemistry allows enhanced interaction with various materials, providing advantages in composite fabrication over graphene's relatively inert surface. While graphene offers exceptional mechanical strength and thermal stability, MXenes' versatility in functionalization presents a broader range of functional applications in materials engineering.
Table of Comparison
| Property | MXenes | Graphene |
|---|---|---|
| Composition | Transition metal carbides, nitrides, or carbonitrides | Single layer of carbon atoms in hexagonal lattice |
| Electrical Conductivity | High, tunable via surface terminations | Extremely high, up to 10^6 S/m |
| Surface Area | 800-1500 m2/g | ~2630 m2/g |
| Mechanical Strength | Good, flexible composites | Exceptional tensile strength (~130 GPa) |
| Thermal Conductivity | Moderate, influenced by defects | Very high (~5000 W/m*K) |
| Hydrophilicity | Hydrophilic due to surface groups | Hydrophobic, requires functionalization |
| Applications | Energy storage, sensors, catalysis, EMI shielding | Electronics, composites, batteries, sensors |
| Stability | Prone to oxidation in air | Highly stable under ambient conditions |
Introduction to MXenes and Graphene
MXenes, a class of 2D transition metal carbides, nitrides, or carbonitrides, exhibit exceptional electrical conductivity, hydrophilicity, and mechanical flexibility, making them promising for energy storage and sensing applications. Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, is renowned for its extraordinary strength, high electron mobility, and thermal conductivity. Both materials offer unique surface chemistries and electronic properties, enabling diverse applications in electronics, catalysis, and composite materials.
Structural Comparison: MXenes vs Graphene
MXenes exhibit a layered structure composed of transition metal carbides, nitrides, or carbonitrides, characterized by their two-dimensional sheets terminated with functional groups like -OH, -O, or -F, which enhance their surface chemistry and hydrophilicity. Graphene features a single layer of sp2-bonded carbon atoms arranged in a hexagonal lattice, providing exceptional electrical conductivity and mechanical strength but lacking intrinsic surface terminations. The presence of surface functional groups in MXenes enables superior chemical versatility and tunable properties compared to the pristine, non-functionalized basal plane of graphene.
Synthesis Methods and Scalability
MXenes are typically synthesized through selective etching of MAX phases using hydrofluoric acid or fluoride-containing mixtures, enabling layer-by-layer exfoliation suitable for mass production, while graphene is primarily produced via chemical vapor deposition (CVD), mechanical exfoliation, or chemical reduction of graphene oxide, with CVD offering higher scalability but higher costs. MXene synthesis benefits from relatively lower temperatures and straightforward chemical processes, leading to better control over surface terminations that influence material properties. Scalability of MXenes is enhanced by solution-based processing, making them advantageous for large-scale energy storage and sensor applications compared to the more complex and costly graphene synthesis routes.
Electrical and Optical Properties
MXenes exhibit superior electrical conductivity compared to graphene due to their metallic nature and tunable surface terminations, enabling enhanced electron transport in energy storage and sensor applications. Graphene's optical properties feature exceptional transparency and broadband absorption, making it ideal for photonic devices and optoelectronics. MXenes also offer adjustable plasmonic resonances and strong light-matter interactions, providing versatile functionalities beyond graphene's capabilities in electromagnetic interference shielding and photodetection.
Mechanical Strength and Flexibility
MXenes exhibit superior mechanical strength and flexibility compared to graphene due to their layered transition metal carbides or nitrides structure, enabling enhanced tensile strength and bending resilience. The presence of surface functional groups in MXenes contributes to improved interlayer bonding, resulting in greater mechanical durability under strain. Graphene, while renowned for its exceptional in-plane strength and flexibility, often faces limitations in out-of-plane deformation and interlayer cohesion, making MXenes a promising alternative for advanced flexible electronics and structural applications.
Thermal Conductivity and Stability
MXenes exhibit superior thermal stability compared to graphene, maintaining performance at elevated temperatures due to their robust transition metal carbide structure. While graphene demonstrates exceptional in-plane thermal conductivity, often exceeding 3000 W/mK, MXenes offer more isotropic heat dissipation with conductivity values typically ranging between 20-100 W/mK. The intrinsic oxidation resistance of MXenes enhances their longevity in high-temperature environments, making them preferable for applications requiring sustained thermal management and chemical durability.
Surface Chemistry and Functionalization
MXenes exhibit diverse surface chemistries due to their abundant surface terminations like hydroxyl, oxygen, and fluorine groups, enabling versatile functionalization compared to graphene's relatively inert sp2 carbon lattice. These terminal groups on MXenes facilitate enhanced hydrophilicity, tunable electronic properties, and improved compatibility with various polymers and biomolecules, making MXenes highly adaptable for sensors, energy storage, and catalysis. Graphene functionalization often requires defect introduction or chemical modification, limiting surface versatility, whereas MXenes' intrinsic surface terminations provide easier and more controlled chemical modification pathways.
Applications in Energy Storage Devices
MXenes exhibit superior electrical conductivity and hydrophilicity compared to graphene, enhancing ion transport and storage in supercapacitors and lithium-ion batteries. Their tunable surface chemistry allows for improved electrode-electrolyte interaction, leading to higher capacitance and energy density. Graphene, while offering exceptional conductivity and mechanical strength, faces challenges in restacking and limited active sites, making MXenes more versatile for next-generation energy storage applications.
Environmental and Biomedical Prospects
MXenes exhibit exceptional electrochemical properties and hydrophilicity, making them highly promising for environmental applications like water purification and heavy metal ion removal, outperforming graphene's largely hydrophobic surface. In biomedical fields, MXenes' biocompatibility and photothermal conversion efficiency enable advancements in drug delivery and cancer therapy, whereas graphene's biocompatibility challenges limit its clinical use. The tunable surface chemistry and unique two-dimensional layered structure of MXenes facilitate enhanced biofunctionalization and environmental remediation compared to graphene.
Future Outlook and Research Challenges
MXenes exhibit superior hydrophilicity and tunable surface chemistry compared to graphene, positioning them as promising materials for energy storage, sensors, and catalysis research. Future outlook emphasizes overcoming challenges in large-scale synthesis and improving oxidation stability to fully exploit MXenes' potential in flexible electronics and environmental applications. Research efforts continue to address integration difficulties and long-term durability to advance both MXenes and graphene in next-generation nanodevices.
2D nanomaterials
MXenes exhibit superior electrical conductivity and hydrophilicity compared to graphene, making them highly versatile 2D nanomaterials for energy storage and sensing applications.
Transition metal carbides
Transition metal carbide MXenes exhibit superior electrical conductivity and tunable surface chemistry compared to graphene, making them highly effective for energy storage and catalytic applications.
Surface functionalization
MXenes exhibit superior surface functionalization versatility compared to graphene due to their abundant surface terminations like -OH, -O, and -F, enabling enhanced chemical reactivity and tunable electronic properties.
Electrical conductivity
MXenes exhibit higher electrical conductivity than graphene due to their metallic nature and surface terminations that enhance electron mobility.
Layered structure
MXenes exhibit a versatile layered structure composed of transition metal carbides, nitrides, or carbonitrides interleaved with surface terminations, while graphene features a single-atom-thick, hexagonally arranged carbon layer providing exceptional electrical conductivity and mechanical strength.
Intercalation chemistry
MXenes exhibit superior intercalation chemistry compared to graphene due to their layered structure, tunable surface terminations, and higher volumetric capacitance enabling enhanced ion storage and fast charge-discharge cycles.
Electrocatalysis
MXenes exhibit superior electrocatalytic performance compared to graphene due to their tunable surface chemistry, high electrical conductivity, and abundant active sites.
Energy storage
MXenes exhibit superior volumetric capacitance and faster ion intercalation rates compared to graphene, making them more effective for high-performance energy storage applications.
Mechanical exfoliation
Mechanical exfoliation of MXenes produces versatile 2D materials with superior structural flexibility and tunable surface chemistry compared to graphene.
Hydrophilicity
MXenes exhibit superior hydrophilicity compared to graphene due to their abundant surface functional groups, enhancing their dispersion in aqueous environments and enabling broader applications in sensing and energy storage.
MXenes vs Graphene Infographic
 njnir.com
njnir.com