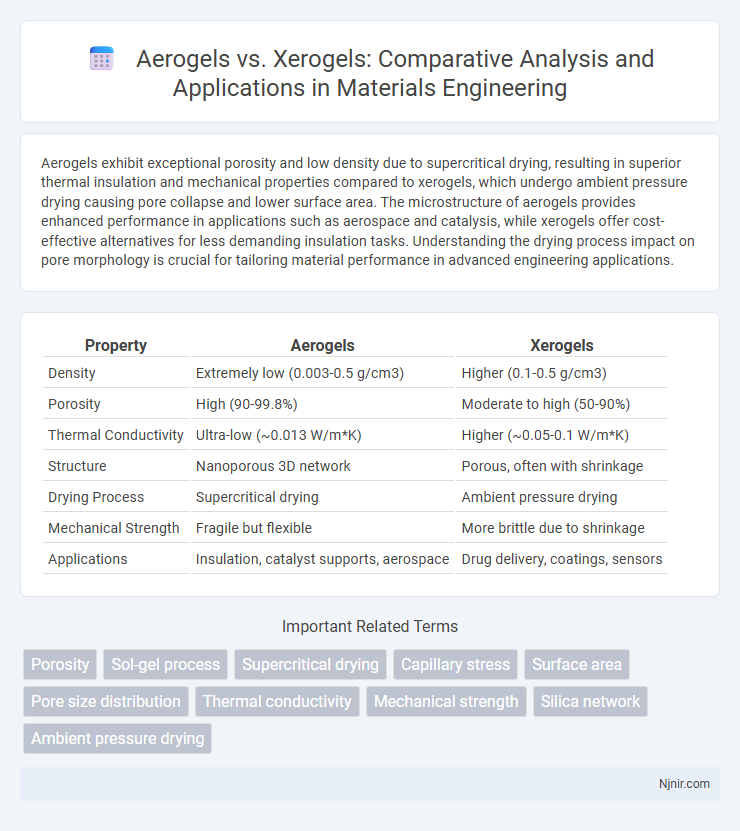
Aerogels exhibit exceptional porosity and low density due to supercritical drying, resulting in superior thermal insulation and mechanical properties compared to xerogels, which undergo ambient pressure drying causing pore collapse and lower surface area. The microstructure of aerogels provides enhanced performance in applications such as aerospace and catalysis, while xerogels offer cost-effective alternatives for less demanding insulation tasks. Understanding the drying process impact on pore morphology is crucial for tailoring material performance in advanced engineering applications.
Table of Comparison
| Property | Aerogels | Xerogels |
|---|---|---|
| Density | Extremely low (0.003-0.5 g/cm3) | Higher (0.1-0.5 g/cm3) |
| Porosity | High (90-99.8%) | Moderate to high (50-90%) |
| Thermal Conductivity | Ultra-low (~0.013 W/m*K) | Higher (~0.05-0.1 W/m*K) |
| Structure | Nanoporous 3D network | Porous, often with shrinkage |
| Drying Process | Supercritical drying | Ambient pressure drying |
| Mechanical Strength | Fragile but flexible | More brittle due to shrinkage |
| Applications | Insulation, catalyst supports, aerospace | Drug delivery, coatings, sensors |
Introduction to Aerogels and Xerogels
Aerogels are ultra-light, highly porous materials derived from gels where the liquid component is replaced with gas, resulting in exceptional insulation and low density. Xerogels, in contrast, are formed by drying gels under ambient conditions, causing significant shrinkage and a denser, less porous structure compared to aerogels. Both materials find applications in thermal insulation, catalysts, and sensors, but their differing drying processes define their unique physical properties and performance.
Historical Development and Discovery
Aerogels were first developed in the 1930s by Samuel Kistler, who replaced the liquid in a gel with gas without causing shrinkage, pioneering the field of lightweight, porous materials. Xerogels originated earlier through traditional drying methods, resulting in denser, less porous structures due to liquid evaporation at ambient pressure. These distinct historical developments highlight the transition from xerogel production, characterized by shrinkage and pore collapse, to the advanced supercritical drying techniques that enabled the creation of highly porous aerogels.
Synthesis and Fabrication Methods
Aerogels are synthesized primarily through supercritical drying, which preserves their porous network by avoiding capillary stress, while xerogels undergo ambient pressure drying that often results in shrinkage and reduced porosity. The fabrication of aerogels involves sol-gel processes followed by supercritical fluid extraction, ensuring high surface area and low density. In contrast, xerogels are produced via conventional drying methods after gelation, leading to denser materials with lower thermal insulation properties.
Structural and Morphological Differences
Aerogels exhibit an ultra-low density and high porosity with a nanoporous structure formed through supercritical drying, preserving the gel network and resulting in a highly interconnected three-dimensional framework. Xerogels, on the other hand, undergo evaporative drying, causing significant shrinkage and collapse of the porous network, which yields a denser, less porous material with reduced surface area and larger pore sizes. The primary structural difference lies in the preservation of the porous morphology in aerogels compared to the denser, compact morphology characteristic of xerogels.
Mechanical Properties Comparison
Aerogels exhibit exceptional mechanical strength and low density due to their highly porous, three-dimensional network structure, while xerogels tend to be denser with more brittle characteristics resulting from shrinkage during drying. The compressive modulus of aerogels typically ranges from 0.1 to 1 MPa, much higher than xerogels, which often fall below 0.1 MPa due to their lower porosity. Aerogels also maintain superior resilience and flexibility under mechanical stress, making them preferable for applications requiring lightweight yet mechanically robust materials.
Thermal and Acoustic Insulation Performance
Aerogels exhibit superior thermal insulation due to their ultra-low density and nanoporous structure, resulting in thermal conductivity values as low as 0.013 W/m*K, outperforming xerogels which typically have higher density and thermal conductivity. In acoustic insulation, aerogels provide excellent sound absorption, especially in the low-frequency range, thanks to their open-cell morphology, whereas xerogels tend to be less effective due to denser and more brittle frameworks. The unique structural properties of aerogels make them ideal for advanced insulation applications requiring minimal thermal bridging and enhanced noise reduction.
Surface Chemistry and Porosity
Aerogels exhibit extremely high porosity with pore sizes typically ranging from 10 to 100 nanometers, resulting in surface areas often exceeding 600 m2/g, whereas xerogels tend to have lower porosity and smaller surface areas due to pore collapse during drying. The surface chemistry of aerogels is characterized by abundant silanol groups (Si-OH) on the silica network, enhancing hydrophilicity and enabling functionalization, while xerogels exhibit fewer accessible silanol groups due to densification. These differences in surface chemistry and porosity directly influence adsorption capacity, thermal insulation properties, and catalytic performance between aerogels and xerogels.
Applications in Industry and Technology
Aerogels, characterized by their ultra-low density and high porosity, find extensive applications in thermal insulation for aerospace, construction, and oil and gas industries due to their exceptional heat resistance and lightweight properties. Xerogels, formed through evaporative drying, offer advantages in catalysis, sensors, and drug delivery systems, benefiting from their porous structure and mechanical stability. Both materials enhance advancements in environmental technology, with aerogels excelling in superinsulation and xerogels improving the efficiency of adsorption and filtration processes.
Environmental Impact and Sustainability
Aerogels, known for their ultra-lightweight and high porosity, offer superior insulation properties that reduce energy consumption in buildings, contributing to lower greenhouse gas emissions. Xerogels, while also porous, typically require solvents that pose environmental disposal challenges, leading to greater ecological impact during production. The sustainable advantage of aerogels lies in their long lifespan and energy efficiency, making them a more eco-friendly option in green technology applications.
Future Trends and Research Directions
Emerging research on aerogels and xerogels emphasizes enhancing mechanical strength and thermal insulation through nanostructure optimization and hybrid material integration. Future trends highlight scalable production techniques, including eco-friendly synthesis methods aiming to reduce costs and environmental impact. Innovations also focus on functionalizing aerogels and xerogels for applications in energy storage, environmental remediation, and wearable technology.
Porosity
Aerogels exhibit higher porosity up to 99.8% compared to xerogels, which typically have lower porosity due to shrinkage during ambient pressure drying.
Sol-gel process
The sol-gel process transforms solutions into rigid aerogels through supercritical drying, preserving porous nanostructures, whereas xerogels result from ambient drying, causing significant shrinkage and pore collapse.
Supercritical drying
Aerogels achieve superior porosity and minimal shrinkage through supercritical drying that prevents capillary stress, whereas xerogels undergo ambient drying causing pore collapse and higher density.
Capillary stress
Aerogels exhibit significantly lower capillary stress than xerogels due to their supercritical drying process, which prevents pore collapse and preserves high porosity and surface area.
Surface area
Aerogels typically exhibit a significantly higher surface area, often exceeding 600 m2/g, compared to xerogels whose surface area usually ranges between 100 and 300 m2/g due to differences in drying methods affecting pore structure.
Pore size distribution
Aerogels typically exhibit a narrower and more uniform pore size distribution ranging from 1 to 100 nanometers, while xerogels often have a broader and less controlled pore size distribution due to shrinkage during drying.
Thermal conductivity
Aerogels exhibit significantly lower thermal conductivity ranging from 0.013 to 0.03 W/m*K compared to xerogels, which typically have higher values around 0.05 to 0.1 W/m*K, making aerogels superior insulators.
Mechanical strength
Aerogels exhibit higher mechanical strength than xerogels due to their porous, lightweight structure formed through supercritical drying, which preserves the gel network and minimizes shrinkage.
Silica network
Aerogels feature a highly porous, low-density silica network formed through supercritical drying, while xerogels possess a denser silica structure created by ambient pressure drying that induces network shrinkage and pore collapse.
Ambient pressure drying
Aerogels produced via ambient pressure drying retain superior porosity and lower density compared to xerogels, which often exhibit shrinkage and decreased pore volume due to capillary stress during the drying process.
Aerogels vs Xerogels Infographic
 njnir.com
njnir.com