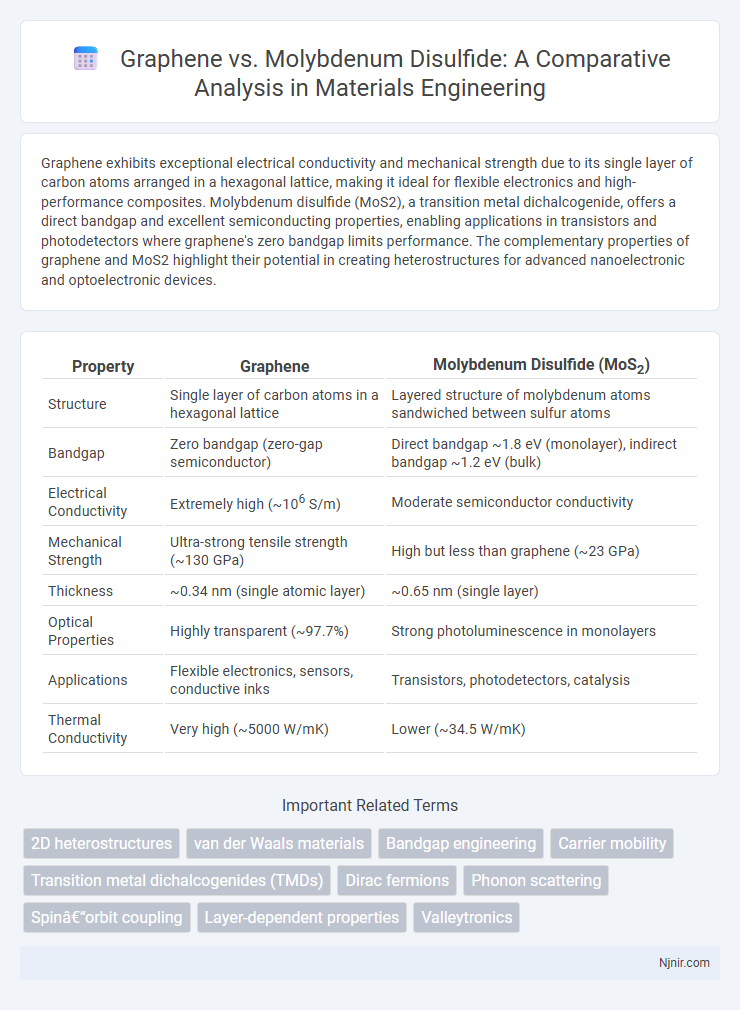
Graphene exhibits exceptional electrical conductivity and mechanical strength due to its single layer of carbon atoms arranged in a hexagonal lattice, making it ideal for flexible electronics and high-performance composites. Molybdenum disulfide (MoS2), a transition metal dichalcogenide, offers a direct bandgap and excellent semiconducting properties, enabling applications in transistors and photodetectors where graphene's zero bandgap limits performance. The complementary properties of graphene and MoS2 highlight their potential in creating heterostructures for advanced nanoelectronic and optoelectronic devices.
Table of Comparison
| Property | Graphene | Molybdenum Disulfide (MoS2) |
|---|---|---|
| Structure | Single layer of carbon atoms in a hexagonal lattice | Layered structure of molybdenum atoms sandwiched between sulfur atoms |
| Bandgap | Zero bandgap (zero-gap semiconductor) | Direct bandgap ~1.8 eV (monolayer), indirect bandgap ~1.2 eV (bulk) |
| Electrical Conductivity | Extremely high (~106 S/m) | Moderate semiconductor conductivity |
| Mechanical Strength | Ultra-strong tensile strength (~130 GPa) | High but less than graphene (~23 GPa) |
| Thickness | ~0.34 nm (single atomic layer) | ~0.65 nm (single layer) |
| Optical Properties | Highly transparent (~97.7%) | Strong photoluminescence in monolayers |
| Applications | Flexible electronics, sensors, conductive inks | Transistors, photodetectors, catalysis |
| Thermal Conductivity | Very high (~5000 W/mK) | Lower (~34.5 W/mK) |
Introduction to Graphene and Molybdenum Disulfide
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, renowned for its exceptional electrical conductivity, mechanical strength, and thermal properties. Molybdenum disulfide (MoS2) is a transition metal dichalcogenide with a layered structure, known for its semiconducting behavior, appreciable bandgap, and applications in photodetectors and flexible electronics. Both materials exhibit unique electronic and mechanical characteristics that make them promising candidates for next-generation nanoelectronics and optoelectronic devices.
Atomic Structure and Layered Morphologies
Graphene consists of a single layer of carbon atoms arranged in a hexagonal lattice, exhibiting a planar atomic structure that enables exceptional electrical conductivity and mechanical strength. Molybdenum disulfide (MoS2) features a layered morphology with molybdenum atoms sandwiched between sulfur atoms, forming a trigonal prismatic coordination within each monolayer, which imparts semiconducting properties. The van der Waals forces between MoS2 layers allow mechanical exfoliation into few-layer or monolayer forms, significantly influencing its electronic band structure compared to graphene's zero bandgap.
Synthesis Methods and Scalability
Graphene, primarily synthesized through chemical vapor deposition (CVD) and mechanical exfoliation, offers scalability but faces challenges in uniformity and defect control, limiting large-scale industrial applications. Molybdenum disulfide (MoS2) synthesis utilizes liquid-phase exfoliation and sulfurization of molybdenum oxides, with chemical vapor deposition emerging as a scalable method producing high-quality monolayers. While graphene benefits from more established large-area synthesis techniques, MoS2 requires further optimization in scalable production to achieve consistent material quality for electronics and optoelectronic applications.
Electronic and Optical Properties Comparison
Graphene exhibits exceptional electronic properties such as ultra-high electron mobility exceeding 200,000 cm2/V*s and zero bandgap, making it ideal for high-speed transistors but limiting its use in digital electronics. In contrast, molybdenum disulfide (MoS2) presents a direct bandgap of approximately 1.8 eV in its monolayer form, enabling efficient semiconducting behavior essential for optoelectronic devices like photodetectors and transistors with strong current on/off ratios. Optically, MoS2 shows pronounced excitonic peaks and strong photoluminescence, whereas graphene's optical absorption is broadband and relatively featureless due to its zero bandgap nature.
Mechanical Strength and Flexibility
Graphene exhibits exceptional mechanical strength with a tensile strength of approximately 130 GPa and a Young's modulus around 1 TPa, making it one of the strongest materials known. Molybdenum disulfide (MoS2), while less strong with a tensile strength near 23 GPa and a lower Young's modulus of approximately 270 GPa, offers superior flexibility due to its layered structure and ability to undergo significant strain without breaking. These properties make graphene ideal for applications requiring high strength, whereas MoS2 is preferred in flexible electronics where mechanical flexibility is crucial.
Thermal Conductivity and Stability
Graphene exhibits exceptional thermal conductivity, reaching up to 5000 W/mK, making it one of the best heat conductors known. Molybdenum disulfide (MoS2), with a thermal conductivity around 85 W/mK, offers significantly lower heat conduction but provides superior chemical and thermal stability under extreme conditions. The robust stability of MoS2 at high temperatures and in harsh environments contrasts with graphene's tendency to oxidize and degrade, limiting its applications in thermal management where durability is critical.
Chemical Reactivity and Functionalization
Graphene exhibits chemical inertness due to its stable sp2-hybridized carbon lattice, allowing limited functionalization primarily through defect sites or edges. Molybdenum disulfide (MoS2), with its layered transition metal dichalcogenide structure, presents higher chemical reactivity, enabling diverse functionalization via sulfur vacancies and transition metal sites. The ability to chemically modify MoS2 facilitates applications in catalysis and sensing, whereas graphene's functionalization targets enhanced electronic properties and compatibility with organic molecules.
Applications in Electronics and Energy Storage
Graphene exhibits exceptional electrical conductivity and mechanical strength, making it ideal for flexible electronics, high-speed transistors, and supercapacitors with rapid charge-discharge cycles. Molybdenum disulfide offers a direct bandgap and strong light-matter interaction, enabling its use in semiconducting devices, photodetectors, and efficient lithium-ion battery anodes. Both materials contribute significantly to advancing energy storage systems, with graphene enhancing electrode conductivity and MoS2 improving charge capacity and stability.
Environmental Impact and Sustainability
Graphene offers remarkable environmental benefits due to its potential for energy-efficient production methods and its ability to improve renewable energy technologies like solar cells and water purification. Molybdenum disulfide, while also useful in sustainable applications such as catalysis and lubrication, requires mining processes that can have significant ecological footprints if not managed responsibly. Overall, graphene's scalable synthesis from abundant carbon sources presents a more sustainable path compared to molybdenum disulfide's reliance on less abundant materials and more intensive extraction methods.
Future Prospects in Materials Engineering
Graphene exhibits exceptional electrical conductivity and mechanical strength, positioning it as a groundbreaking material for future flexible electronics and high-performance composites. Molybdenum disulfide (MoS2) presents unique semiconducting properties and a direct bandgap ideal for next-generation optoelectronic devices and sensors. Advancements in hybrid structures combining graphene and MoS2 promise innovative applications in energy storage, nanoelectronics, and photodetectors, driving transformative progress in materials engineering.
2D heterostructures
Graphene and molybdenum disulfide form 2D heterostructures that combine graphene's high electrical conductivity with molybdenum disulfide's semiconducting properties, enabling advanced applications in nanoelectronics and optoelectronics.
van der Waals materials
Graphene and molybdenum disulfide are prominent van der Waals materials distinguished by graphene's exceptional electrical conductivity and mechanical strength versus molybdenum disulfide's intrinsic semiconducting properties and strong spin-orbit coupling.
Bandgap engineering
Graphene's zero bandgap limits its semiconductor applications, whereas molybdenum disulfide's intrinsic 1.8 eV direct bandgap enables efficient bandgap engineering for optoelectronic devices.
Carrier mobility
Graphene exhibits exceptionally high carrier mobility exceeding 200,000 cm2/V*s, while molybdenum disulfide's carrier mobility is significantly lower, typically around 200 cm2/V*s.
Transition metal dichalcogenides (TMDs)
Transition metal dichalcogenides (TMDs) like molybdenum disulfide offer tunable bandgaps and strong spin-orbit coupling, providing versatile electronic and optoelectronic properties that complement graphene's exceptional conductivity and zero bandgap characteristics.
Dirac fermions
Graphene exhibits massless Dirac fermions with linear energy dispersion enabling exceptional electron mobility, whereas molybdenum disulfide hosts massive Dirac fermions due to its sizable bandgap resulting in lower carrier mobility.
Phonon scattering
Phonon scattering in graphene is significantly lower than in molybdenum disulfide due to graphene's superior lattice symmetry and stronger carbon-carbon bonds, resulting in higher thermal conductivity and more efficient heat dissipation.
Spin–orbit coupling
Graphene exhibits weak spin-orbit coupling due to its light carbon atoms, whereas molybdenum disulfide demonstrates strong spin-orbit coupling originating from heavy molybdenum atoms and lack of inversion symmetry.
Layer-dependent properties
Graphene exhibits exceptional electrical conductivity and mechanical strength that vary minimally with layer number, while molybdenum disulfide demonstrates a pronounced layer-dependent transition from an indirect bandgap in bulk to a direct bandgap in monolayers, significantly affecting its optical and electronic properties.
Valleytronics
Graphene's high carrier mobility contrasts with molybdenum disulfide's direct bandgap and strong spin-orbit coupling, making MoS2 more effective for manipulating valley pseudospins in valleytronics applications.
graphene vs molybdenum disulfide Infographic
 njnir.com
njnir.com