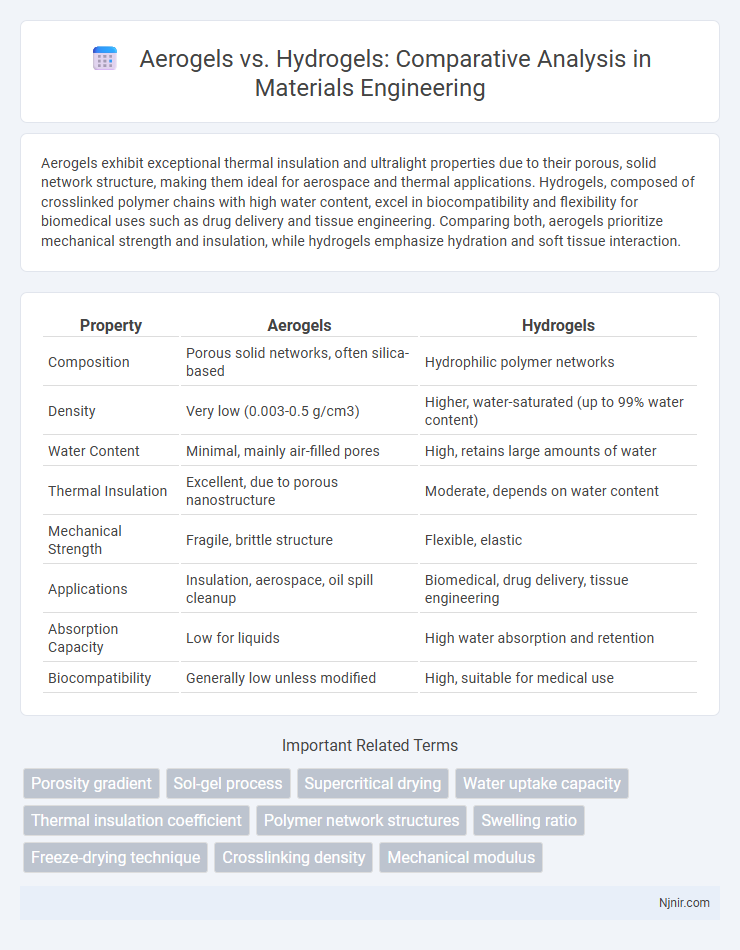
Aerogels exhibit exceptional thermal insulation and ultralight properties due to their porous, solid network structure, making them ideal for aerospace and thermal applications. Hydrogels, composed of crosslinked polymer chains with high water content, excel in biocompatibility and flexibility for biomedical uses such as drug delivery and tissue engineering. Comparing both, aerogels prioritize mechanical strength and insulation, while hydrogels emphasize hydration and soft tissue interaction.
Table of Comparison
| Property | Aerogels | Hydrogels |
|---|---|---|
| Composition | Porous solid networks, often silica-based | Hydrophilic polymer networks |
| Density | Very low (0.003-0.5 g/cm3) | Higher, water-saturated (up to 99% water content) |
| Water Content | Minimal, mainly air-filled pores | High, retains large amounts of water |
| Thermal Insulation | Excellent, due to porous nanostructure | Moderate, depends on water content |
| Mechanical Strength | Fragile, brittle structure | Flexible, elastic |
| Applications | Insulation, aerospace, oil spill cleanup | Biomedical, drug delivery, tissue engineering |
| Absorption Capacity | Low for liquids | High water absorption and retention |
| Biocompatibility | Generally low unless modified | High, suitable for medical use |
Introduction to Aerogels and Hydrogels
Aerogels are ultralight, porous materials derived from gels in which the liquid component is replaced by gas without collapsing the solid matrix, resulting in exceptional thermal insulation and low density. Hydrogels consist of three-dimensional polymer networks capable of absorbing and retaining significant amounts of water, making them ideal for biomedical applications and tissue engineering. Both materials exhibit unique structural properties that cater to diverse industrial and scientific uses, with aerogels excelling in insulation and hydrogels in moisture retention and biocompatibility.
Structural Differences: Aerogels vs Hydrogels
Aerogels possess a highly porous, rigid, and lightweight structure composed mainly of silica or carbon, resulting in low density and high surface area suitable for insulation and adsorption applications. Hydrogels feature a three-dimensional polymer network swollen with water, providing softness, flexibility, and the ability to retain large amounts of moisture for biomedical and drug delivery uses. The key structural difference lies in aerogels having a solid, dry framework with air-filled pores, whereas hydrogels consist of hydrated polymer chains forming a gel-like, elastic material.
Synthesis Methods and Chemical Composition
Aerogels are synthesized primarily through sol-gel processes followed by supercritical drying, resulting in highly porous, silica-based or polymeric networks with low density and high surface area. Hydrogels are formed via crosslinking hydrophilic polymers such as polyacrylamide or polyethylene glycol, using chemical or physical methods that create a three-dimensional, water-absorbing polymer network. The chemical composition of aerogels typically involves inorganic oxides like SiO2 or metal oxides, whereas hydrogels are composed mainly of organic polymers capable of retaining significant amounts of water.
Mechanical Properties Comparison
Aerogels exhibit remarkable compressive strength and elasticity due to their highly porous, nanostructured frameworks, making them lightweight yet mechanically robust. Hydrogels, composed of crosslinked polymer networks swollen with water, offer superior flexibility and tensile strength but generally suffer from lower compressive strength compared to aerogels. The mechanical properties of aerogels favor applications requiring high insulation and structural integrity, while hydrogels are better suited for environments demanding biocompatibility and deformability.
Thermal and Electrical Conductivity
Aerogels exhibit exceptional thermal insulation due to their highly porous, low-density structure, with thermal conductivity as low as 0.013 W/m*K, making them ideal for advanced thermal management applications. Hydrogels, composed mainly of water, have significantly higher thermal conductivity, typically around 0.5 W/m*K, limiting their effectiveness as thermal insulators but allowing efficient heat retention in biomedical uses. Electrically, aerogels made with conductive materials can achieve enhanced conductivity suitable for sensors and energy storage, whereas hydrogels generally possess low electrical conductivity unless doped with conductive polymers or nanoparticles.
Applications in Industry and Technology
Aerogels exhibit exceptional thermal insulation properties, making them ideal for aerospace, construction, and oil and gas industries where lightweight and high-performance insulation is critical. Hydrogels are widely used in biomedical applications, such as drug delivery systems, wound dressings, and tissue engineering, due to their high water content and biocompatibility. Both aerogels and hydrogels find roles in environmental technology, with aerogels used for pollutant adsorption and hydrogels for water purification and controlled release of agrochemicals.
Environmental Impact and Sustainability
Aerogels exhibit exceptional thermal insulation and lightweight properties, making them highly efficient for reducing energy consumption in construction and industrial applications, which contributes to lower carbon emissions. Hydrogels, composed primarily of water and biodegradable polymers, support sustainable agriculture by enhancing soil moisture retention and reducing the need for frequent irrigation and chemical fertilizers. Both materials present eco-friendly benefits, but aerogels' manufacturing processes often consume more energy compared to hydrogels, making lifecycle assessments critical for determining overall environmental impact.
Advances in Hybrid Aerogel-Hydrogel Materials
Hybrid aerogel-hydrogel materials combine the ultralight, porous structure of aerogels with the high water retention and flexibility of hydrogels, enabling advances in biomedical applications and environmental technologies. Recent developments emphasize enhanced mechanical strength, tunable porosity, and improved biocompatibility, making these hybrid composites ideal for drug delivery, tissue engineering, and water purification. Innovations in fabrication techniques such as freeze-drying and sol-gel processes have facilitated controlled hybrid material properties, expanding their multifunctional potential.
Challenges in Manufacturing and Scalability
Aerogels face significant manufacturing challenges due to their fragile nanostructure and the requirement for supercritical drying, which increases production costs and limits scalability. Hydrogels, while easier to produce through conventional polymerization methods, encounter difficulties in maintaining mechanical strength and uniformity at large scales. Both materials require advancements in fabrication techniques to overcome these barriers and enable widespread industrial applications.
Future Perspectives in Materials Engineering
Aerogels, with their ultra-low density and exceptional thermal insulation properties, are poised to revolutionize aerospace and energy storage applications by enabling lighter, more efficient structures. Hydrogels' biocompatibility and tunable swelling behaviors offer promising advancements in biomedical engineering, particularly for drug delivery systems and tissue scaffolding. Future materials engineering will increasingly integrate aerogel and hydrogel composites to harness combined advantages, driving innovations in environmental sustainability and smart material technologies.
Porosity gradient
Aerogels exhibit a higher porosity gradient than hydrogels, enabling superior insulation and lightweight structural properties.
Sol-gel process
Aerogels and hydrogels are both synthesized via the sol-gel process, where aerogels undergo supercritical drying to create a porous, ultra-low-density solid, while hydrogels retain water within a three-dimensional polymer network formed during gelation.
Supercritical drying
Supercritical drying preserves the porous nanostructure of aerogels by eliminating surface tension during solvent removal, unlike hydrogels which typically undergo conventional drying that collapses their network.
Water uptake capacity
Aerogels exhibit significantly lower water uptake capacity compared to hydrogels due to their highly porous, hydrophobic structure versus the hydrophilic, cross-linked polymer network in hydrogels that enables extensive water absorption.
Thermal insulation coefficient
Aerogels exhibit an exceptionally low thermal insulation coefficient ranging from 0.013 to 0.020 W/m*K, significantly outperforming hydrogels whose thermal insulation typically ranges around 0.5 W/m*K.
Polymer network structures
Aerogels feature highly porous, rigid polymer networks with minimal cross-linking that provide low density and thermal insulation, whereas hydrogels possess hydrated, flexible polymer networks with extensive cross-linking enabling high water absorption and swelling behavior.
Swelling ratio
Aerogels exhibit a low swelling ratio due to their rigid, porous structure, whereas hydrogels demonstrate a high swelling ratio attributed to their hydrophilic polymer networks.
Freeze-drying technique
Freeze-drying preserves the ultra-light porous structure of aerogels by sublimating ice under low temperature and pressure, whereas hydrogels often suffer structural collapse during freeze-drying due to their high water content and flexible polymer networks.
Crosslinking density
Aerogels exhibit lower crosslinking density resulting in higher porosity and lightweight structure, whereas hydrogels have higher crosslinking density that enhances water retention and mechanical strength.
Mechanical modulus
Aerogels exhibit an exceptionally low mechanical modulus due to their highly porous and lightweight structure, while hydrogels possess a significantly higher mechanical modulus attributed to their water-swollen polymer networks.
Aerogels vs Hydrogels Infographic
 njnir.com
njnir.com