Superhydrophobic coatings create surfaces that repel water by forming a high contact angle, preventing moisture accumulation and reducing corrosion risks in materials engineering. Hydrophilic coatings, in contrast, promote water spread and absorption, enhancing adhesion and improving heat dissipation in applications requiring moisture interaction. Understanding the differences between these coatings is crucial for selecting optimal materials that balance water resistance and surface wettability in various engineering environments.
Table of Comparison
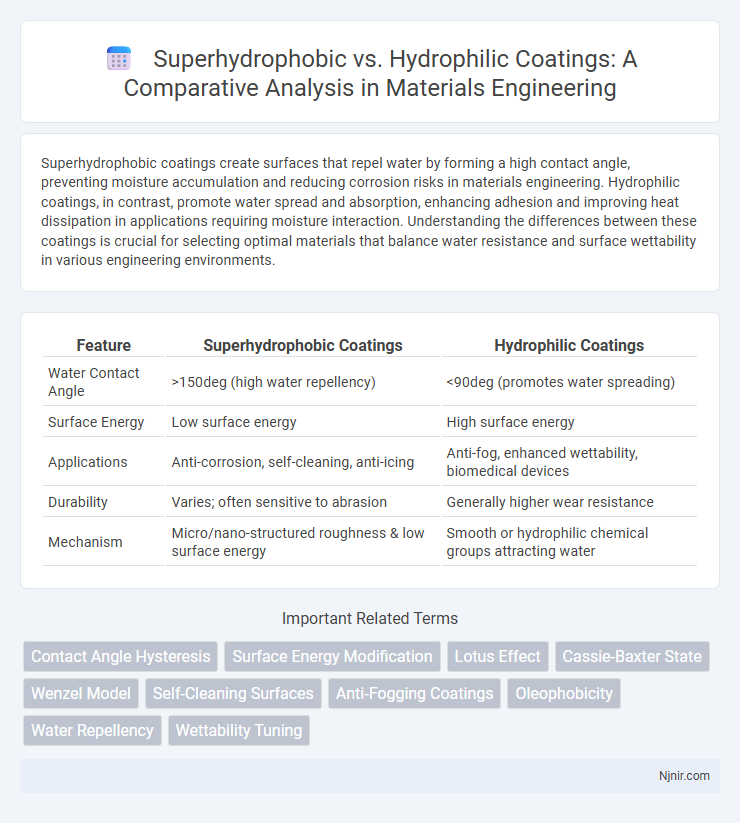
| Feature | Superhydrophobic Coatings | Hydrophilic Coatings |
|---|---|---|
| Water Contact Angle | >150deg (high water repellency) | <90deg (promotes water spreading) |
| Surface Energy | Low surface energy | High surface energy |
| Applications | Anti-corrosion, self-cleaning, anti-icing | Anti-fog, enhanced wettability, biomedical devices |
| Durability | Varies; often sensitive to abrasion | Generally higher wear resistance |
| Mechanism | Micro/nano-structured roughness & low surface energy | Smooth or hydrophilic chemical groups attracting water |
Introduction to Superhydrophobic and Hydrophilic Coatings
Superhydrophobic coatings exhibit water contact angles greater than 150 degrees, creating surfaces that repel water and prevent adhesion, ideal for self-cleaning and anti-corrosion applications. Hydrophilic coatings, characterized by water contact angles below 90 degrees, promote water spreading and absorption, enhancing surface wettability and improving processes like anti-fogging and increased adhesion. Both coating types modify surface energy and texture at the micro- and nanoscale to achieve their distinct water interaction properties, enabling diverse industrial and biomedical uses.
Fundamental Principles: Superhydrophobicity vs. Hydrophilicity
Superhydrophobic coatings exhibit a water contact angle greater than 150 degrees, causing water droplets to bead and roll off surfaces due to trapped air pockets and low surface energy materials. Hydrophilic coatings, in contrast, have a contact angle below 90 degrees, promoting water spread and adsorption through polar or charged surface groups. These fundamental wetting properties arise from differences in surface chemistry and micro/nanostructures that control interfacial water behavior.
Material Composition of Superhydrophobic Coatings
Superhydrophobic coatings primarily consist of materials with low surface energy combined with micro- and nanoscale roughness, such as fluorinated polymers, silicone derivatives, and silica nanoparticles. These coatings rely on hierarchical structures like silica or titanium dioxide nanoparticles embedded in a hydrophobic matrix to create air pockets that repel water effectively. In contrast to hydrophilic coatings that use hydrophilic polymers like polyethylene glycol or cellulose, superhydrophobic materials emphasize surface texture and chemical composition to minimize water adhesion and enhance water repellency.
Chemical Structure and Fabrication of Hydrophilic Coatings
Hydrophilic coatings typically consist of polymers with polar functional groups such as hydroxyl, carboxyl, or amine groups, which promote strong hydrogen bonding with water molecules. Fabrication methods for hydrophilic coatings include layer-by-layer assembly, plasma treatment, and graft polymerization, allowing precise control over surface chemistry and wettability. These coatings enhance surface energy and promote water spreading, contrasting with the low surface energy and rough micro/nanostructure characteristic of superhydrophobic coatings.
Performance Comparison: Water Repellency and Adhesion
Superhydrophobic coatings exhibit exceptional water repellency with contact angles typically above 150deg, causing water droplets to bead up and roll off surfaces, effectively preventing moisture accumulation and corrosion. In contrast, hydrophilic coatings have contact angles below 90deg, promoting water spread and adhesion, which enhances surface wettability and can improve functionalities like cleaning or anti-fogging. The performance difference significantly impacts applications: superhydrophobic surfaces excel in self-cleaning and anti-icing, while hydrophilic coatings are preferred where water adhesion aids operation, such as in biomedical devices or inkjet printing.
Key Applications in Industrial and Consumer Sectors
Superhydrophobic coatings, known for their extreme water repellency and self-cleaning properties, are widely applied in industries such as automotive, aerospace, electronics, and textiles to prevent corrosion, reduce ice formation, and enhance durability. Hydrophilic coatings, which promote water spreading and absorption, find key uses in medical devices, optical lenses, and filtration systems by improving biocompatibility, clarity, and fluid transport efficiency. In consumer sectors, superhydrophobic coatings are popular for stain-resistant fabrics and smartphone screens, while hydrophilic coatings enhance comfort in contact lenses and improve anti-fog performance in eyewear.
Durability and Environmental Resistance
Superhydrophobic coatings exhibit exceptional durability by repelling water, dirt, and chemicals, thereby reducing surface wear and extending material lifespan in harsh environments. Hydrophilic coatings, while promoting water spread and cleaning, often degrade faster under UV exposure and chemical attack, leading to reduced environmental resistance. Advanced formulations of superhydrophobic coatings incorporate nanostructures and fluorinated compounds, significantly enhancing their mechanical stability and resilience against corrosive agents compared to conventional hydrophilic layers.
Challenges in Manufacturing and Scalability
Superhydrophobic coatings face significant manufacturing challenges due to the need for precise nanostructures that ensure water repellency, often requiring complex and costly fabrication techniques such as chemical vapor deposition or laser etching. In contrast, hydrophilic coatings are generally easier to scale, relying on simpler chemical treatments or polymer coatings that enhance surface wettability without intricate nano-patterning. Both types encounter scalability issues related to durability and consistent performance across large surfaces, but superhydrophobic coatings typically demand stricter quality control to maintain their functionality under varied environmental conditions.
Recent Advances and Innovations
Recent advances in superhydrophobic coatings emphasize nanostructured surfaces with enhanced water repellency, self-cleaning capabilities, and durability for industrial and medical applications. Innovations in hydrophilic coatings focus on improving wettability, anti-fogging properties, and biocompatibility using polymer blends and surface modification techniques. Both coating types benefit from eco-friendly materials and scalable fabrication methods, enabling broader commercial adoption.
Future Trends and Research Directions
Superhydrophobic coatings are evolving with advancements in nanotechnology and biomimetic materials to enhance durability and self-cleaning properties, positioning them as leading candidates for anti-fouling and corrosion-resistant applications. Hydrophilic coatings research is increasingly targeting tunable wettability and improved biocompatibility, critical for medical devices and water purification systems. Future trends emphasize multifunctional surfaces combining superhydrophobicity and hydrophilicity to address diverse industrial challenges and environmental sustainability.
Contact Angle Hysteresis
Superhydrophobic coatings exhibit low contact angle hysteresis, typically below 10deg, enhancing water repellency and self-cleaning properties, whereas hydrophilic coatings demonstrate higher contact angle hysteresis, often exceeding 30deg, resulting in stronger water adhesion and spreading.
Surface Energy Modification
Superhydrophobic coatings drastically reduce surface energy to repel water and minimize adhesion, while hydrophilic coatings increase surface energy to enhance water spreading and improve wettability.
Lotus Effect
Superhydrophobic coatings mimic the Lotus Effect by creating micro- and nanostructures that repel water and prevent adhesion, whereas hydrophilic coatings promote water spread and absorption for enhanced surface wetting.
Cassie-Baxter State
Superhydrophobic coatings utilize the Cassie-Baxter state by trapping air pockets beneath water droplets to minimize surface wetting, whereas hydrophilic coatings promote complete surface contact and spreading of water, enhancing adhesion and absorption.
Wenzel Model
The Wenzel model explains that superhydrophobic coatings increase water repellency by amplifying surface roughness and contact angle, whereas hydrophilic coatings reduce it by promoting water spreading through surface energy.
Self-Cleaning Surfaces
Superhydrophobic coatings create self-cleaning surfaces by repelling water and dirt through extreme water contact angles above 150deg, while hydrophilic coatings promote water spread and corrosion resistance but offer less effective self-cleaning properties.
Anti-Fogging Coatings
Superhydrophobic coatings prevent fogging by repelling water droplets through extreme water contact angles above 150deg, whereas hydrophilic coatings combat fog by promoting uniform water film formation with contact angles below 30deg, making superhydrophobic coatings more effective for clear anti-fogging applications.
Oleophobicity
Superhydrophobic coatings exhibit superior oleophobicity by repelling both water and oil, whereas hydrophilic coatings attract water and usually lack effective oil repellency.
Water Repellency
Superhydrophobic coatings exhibit exceptional water repellency by forming micro- and nanoscale surface structures that create high contact angles above 150deg, whereas hydrophilic coatings promote water spreading with contact angles below 90deg, resulting in low water repellency.
Wettability Tuning
Superhydrophobic coatings achieve extreme water repellency with contact angles above 150deg, while hydrophilic coatings promote water spreading with contact angles below 90deg, enabling precise wettability tuning for applications like anti-fouling and enhanced adhesion.
Superhydrophobic Coatings vs Hydrophilic Coatings Infographic
 njnir.com
njnir.com