Conductive polymers exhibit electrical conductivity due to their conjugated double-bond structure, making them ideal for applications in flexible electronics and sensors. Dielectric polymers, by contrast, possess high insulating properties and are used primarily for energy storage and electrical insulation in capacitors and electronic devices. Understanding the distinct molecular architectures and electrical behaviors of these polymers enables material engineers to tailor their properties for specific electronic and photonic applications.
Table of Comparison
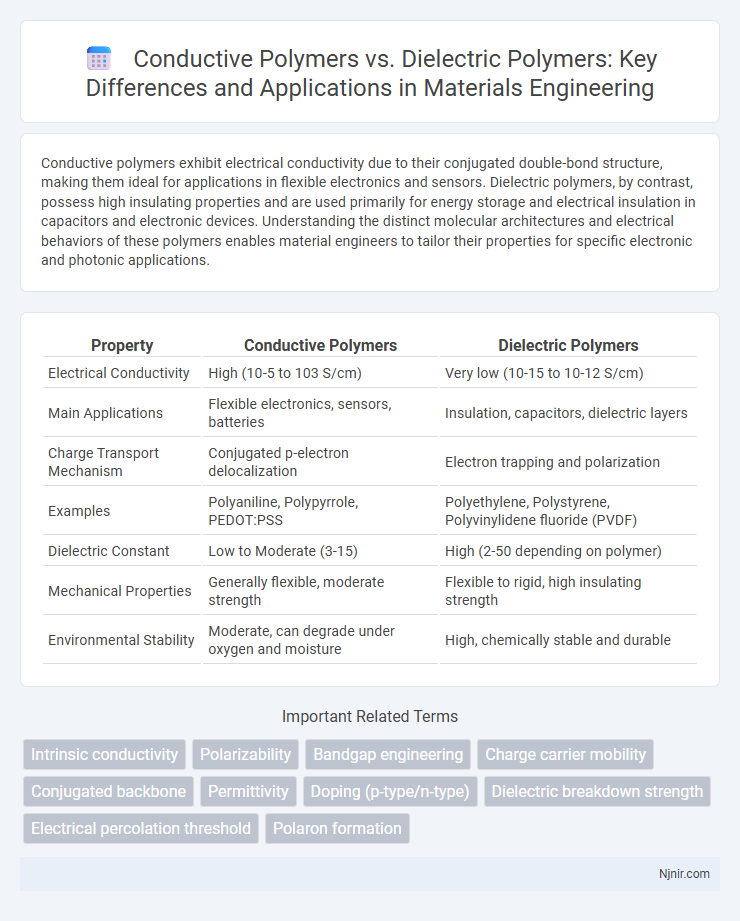
| Property | Conductive Polymers | Dielectric Polymers |
|---|---|---|
| Electrical Conductivity | High (10-5 to 103 S/cm) | Very low (10-15 to 10-12 S/cm) |
| Main Applications | Flexible electronics, sensors, batteries | Insulation, capacitors, dielectric layers |
| Charge Transport Mechanism | Conjugated p-electron delocalization | Electron trapping and polarization |
| Examples | Polyaniline, Polypyrrole, PEDOT:PSS | Polyethylene, Polystyrene, Polyvinylidene fluoride (PVDF) |
| Dielectric Constant | Low to Moderate (3-15) | High (2-50 depending on polymer) |
| Mechanical Properties | Generally flexible, moderate strength | Flexible to rigid, high insulating strength |
| Environmental Stability | Moderate, can degrade under oxygen and moisture | High, chemically stable and durable |
Introduction to Conductive and Dielectric Polymers
Conductive polymers are organic materials that exhibit electrical conductivity due to the presence of conjugated double bonds, enabling electron delocalization along the polymer backbone. Dielectric polymers, on the other hand, are insulating materials characterized by their high dielectric strength and low electrical conductivity, making them ideal for energy storage and insulating applications. These fundamental differences in electronic structure determine their distinct roles in electronics, sensors, and energy devices.
Chemical Structures and Functional Groups
Conductive polymers such as polyaniline, polypyrrole, and polythiophene feature conjugated double bonds along their backbone, allowing pi-electron delocalization that facilitates electrical conductivity. Dielectric polymers like polyethylene, polystyrene, and polyvinyl chloride primarily contain saturated carbon chains or polar functional groups such as chlorides and aromatic rings, which contribute to their high insulating properties by restricting free charge movement. The presence of delocalized pi-electrons in conductive polymers contrasts with the localized sigma bonds and polar groups in dielectric polymers, defining their distinct electrical behaviors.
Mechanisms of Electrical Conductivity
Conductive polymers exhibit electrical conductivity through the movement of charge carriers along conjugated p-electron systems, enabling delocalization of electrons and hopping mechanisms between localized states. In contrast, dielectric polymers function as electrical insulators with their molecular structure lacking free charge carriers, relying on polarization mechanisms such as electronic, ionic, and dipolar polarization to respond to electric fields. The intrinsic differences in their molecular orbital structures define conductive polymers as semiconductors or metals, while dielectric polymers maintain high resistivity and energy storage capacity in capacitive applications.
Synthesis and Processing Techniques
Conductive polymers, such as polyaniline and polythiophene, are synthesized primarily through chemical or electrochemical polymerization techniques, allowing precise control over conductivity and molecular weight. Dielectric polymers like polyethylene and polytetrafluoroethylene are typically produced via free radical polymerization or copolymerization, optimizing their insulating properties and mechanical flexibility. Processing methods for conductive polymers often involve solution casting, spin coating, or vapor phase polymerization to achieve thin films, while dielectric polymers utilize extrusion, molding, and blow molding to form robust insulating layers.
Dielectric Properties and Insulation Performance
Dielectric polymers exhibit high dielectric strength and low electrical conductivity, making them excellent insulators in electronic and electrical applications. Their ability to store and release electrical energy efficiently is characterized by a high dielectric constant and low dielectric loss, crucial for capacitors and insulating materials. Unlike conductive polymers, dielectric polymers maintain insulation performance under high voltage stress, ensuring minimal leakage current and enhanced reliability in power systems.
Applications in Electronics and Energy Storage
Conductive polymers such as polyaniline and polypyrrole are widely used in flexible electronics, sensors, and organic solar cells due to their electrical conductivity and mechanical flexibility. Dielectric polymers like polyethylene terephthalate (PET) and polyvinylidene fluoride (PVDF) are essential in capacitors, insulating layers, and energy storage devices because of their high dielectric constant and excellent insulation properties. The integration of conductive polymers with dielectric polymers enhances the performance of supercapacitors and wearable energy storage systems by combining efficient charge transport with reliable dielectric separation.
Mechanical Properties and Stability
Conductive polymers exhibit moderate mechanical flexibility but often suffer from reduced tensile strength and environmental stability compared to dielectric polymers, which typically possess superior mechanical robustness and long-term chemical and thermal stability. Dielectric polymers such as polyethylene and polytetrafluoroethylene maintain consistent mechanical integrity under stress, while conductive polymers like polyaniline and polypyrrole may degrade or lose conductivity due to oxidation or mechanical fatigue. Advances in composite formulations aim to enhance the mechanical durability and environmental resistance of conductive polymers for reliable performance in flexible electronics and sensing applications.
Environmental Impact and Sustainability
Conductive polymers like polyaniline and polythiophene offer advantages in energy-efficient electronics but often require toxic dopants and complex synthesis processes that challenge environmental sustainability. Dielectric polymers such as polyethylene terephthalate (PET) and polypropylene (PP) provide lower conductivity yet boast greater recyclability and reduced hazardous waste during manufacturing, enhancing their eco-friendly profile. Sustainable polymer design increasingly targets biodegradable and bio-based materials to minimize environmental footprint while balancing electrical performance requirements.
Recent Advances in Polymer Engineering
Recent advances in polymer engineering have revolutionized the development of conductive polymers, enhancing their electrical conductivity and mechanical flexibility through molecular doping and nanocomposite integration. Dielectric polymers have seen significant improvements in energy storage capacity and thermal stability due to the incorporation of high-permittivity fillers and cross-linked polymer networks. Emerging techniques such as molecular design optimization and advanced fabrication methods are driving the tailored performance of both conductive and dielectric polymers for applications in flexible electronics and high-energy capacitors.
Future Trends and Research Directions
Future trends in conductive polymers emphasize enhancing electrical conductivity through molecular doping and nanocomposite integration, targeting flexible electronics and bioelectronic devices. Research on dielectric polymers focuses on improving energy storage capabilities and thermal stability, aiming for advanced capacitors and insulation materials in high-voltage applications. Emerging studies explore hybrid materials combining conductive and dielectric properties to innovate multifunctional devices with tunable electrical characteristics.
Intrinsic conductivity
Conductive polymers exhibit intrinsic conductivity due to their conjugated molecular structure enabling electron delocalization, whereas dielectric polymers lack this conjugation and act as insulators with negligible intrinsic conductivity.
Polarizability
Conductive polymers exhibit lower polarizability compared to dielectric polymers due to their delocalized p-electrons facilitating electrical conductivity rather than dipole alignment under an electric field.
Bandgap engineering
Conductive polymers exhibit tunable narrow bandgaps due to conjugated p-electron systems enabling electrical conductivity, whereas dielectric polymers possess wide bandgaps that prevent charge flow, with bandgap engineering in both focusing on molecular structure modifications to customize electronic and optical properties.
Charge carrier mobility
Conductive polymers exhibit significantly higher charge carrier mobility ranging from 0.1 to 10 cm2/V*s compared to dielectric polymers, which typically have mobility values below 10-8 cm2/V*s, making conductive polymers more efficient for electronic and optoelectronic applications.
Conjugated backbone
Conductive polymers feature a conjugated backbone that enables electron delocalization for electrical conductivity, while dielectric polymers lack conjugation, resulting in insulating properties.
Permittivity
Conductive polymers exhibit lower permittivity due to free charge carriers facilitating conductivity, whereas dielectric polymers possess higher permittivity as their molecular structure stores electrical energy without charge flow.
Doping (p-type/n-type)
Doping conductive polymers with p-type or n-type dopants enhances their electrical conductivity by introducing charge carriers, whereas dielectric polymers remain insulating due to the absence of such doping-induced charge carriers.
Dielectric breakdown strength
Dielectric polymers exhibit significantly higher dielectric breakdown strength, often exceeding 300 MV/m, compared to conductive polymers, making them ideal for insulating applications in high-voltage environments.
Electrical percolation threshold
Conductive polymers exhibit a significantly lower electrical percolation threshold compared to dielectric polymers due to their intrinsic charge transport properties and network formation efficiency.
Polaron formation
Polaron formation in conductive polymers enables charge transport through localized charge carriers coupled with lattice distortion, unlike dielectric polymers which lack free charge carriers and thus do not exhibit polaron-based conductivity.
Conductive polymers vs Dielectric polymers Infographic
 njnir.com
njnir.com