Metal-organic frameworks (MOFs) offer exceptional tunability and higher surface areas compared to zeolites, enabling superior gas storage and separation performance. Zeolites exhibit robust thermal and chemical stability with well-defined microporous structures ideal for catalysis and ion exchange. The choice between MOFs and zeolites depends on application-specific requirements such as structural flexibility, stability, and target molecules.
Table of Comparison
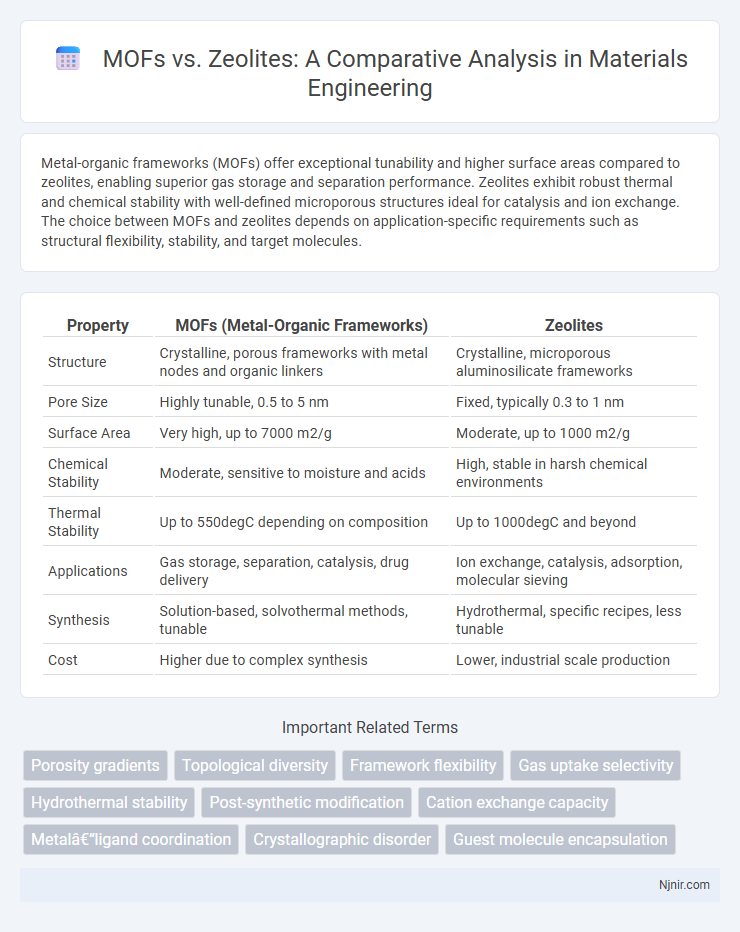
| Property | MOFs (Metal-Organic Frameworks) | Zeolites |
|---|---|---|
| Structure | Crystalline, porous frameworks with metal nodes and organic linkers | Crystalline, microporous aluminosilicate frameworks |
| Pore Size | Highly tunable, 0.5 to 5 nm | Fixed, typically 0.3 to 1 nm |
| Surface Area | Very high, up to 7000 m2/g | Moderate, up to 1000 m2/g |
| Chemical Stability | Moderate, sensitive to moisture and acids | High, stable in harsh chemical environments |
| Thermal Stability | Up to 550degC depending on composition | Up to 1000degC and beyond |
| Applications | Gas storage, separation, catalysis, drug delivery | Ion exchange, catalysis, adsorption, molecular sieving |
| Synthesis | Solution-based, solvothermal methods, tunable | Hydrothermal, specific recipes, less tunable |
| Cost | Higher due to complex synthesis | Lower, industrial scale production |
Introduction to MOFs and Zeolites
Metal-organic frameworks (MOFs) are crystalline materials composed of metal ions coordinated to organic ligands, forming porous structures with high surface area and tunable properties. Zeolites are microporous, aluminosilicate minerals with a well-defined framework of SiO4 and AlO4 tetrahedra, widely used for catalysis and adsorption. Both MOFs and zeolites offer unique advantages in gas storage, separation, and catalysis, with MOFs providing greater structural diversity and zeolites showcasing exceptional stability under harsh conditions.
Structural Differences Between MOFs and Zeolites
Metal-Organic Frameworks (MOFs) possess highly tunable architectures formed by metal ions or clusters coordinated to organic ligands, enabling customizable porosity and functionality. Zeolites feature rigid, crystalline aluminosilicate frameworks with uniform microporous channels and cavities, resulting in exceptional thermal and chemical stability. The key structural difference lies in MOFs' hybrid inorganic-organic composition allowing diverse pore sizes, while zeolites maintain purely inorganic lattices with fixed microporous geometries.
Synthesis Methods: MOFs vs Zeolites
MOFs (Metal-Organic Frameworks) are typically synthesized through solvothermal or hydrothermal methods involving metal ions and organic linkers forming crystalline porous structures. Zeolites, on the other hand, are primarily produced via hydrothermal synthesis using aluminosilicate gels under specific temperature and pH conditions, often requiring mineralizing agents. MOF synthesis offers greater tunability in pore size and functionality due to diverse organic linkers, whereas zeolite synthesis is more constrained by inorganic frameworks but benefits from established industrial scalability and thermal stability.
Surface Area and Porosity Comparisons
Metal-Organic Frameworks (MOFs) exhibit significantly higher surface areas, often exceeding 7,000 m2/g, compared to zeolites, which typically range between 300 and 1,000 m2/g. MOFs possess tunable porosity with pore sizes adjustable from microporous to mesoporous scales, whereas zeolites generally have fixed microporous structures with pore diameters under 2 nm. This enhanced surface area and flexible porosity make MOFs superior candidates for gas storage, separation, and catalysis applications requiring customized adsorption properties.
Thermal and Chemical Stability
Metal-Organic Frameworks (MOFs) generally exhibit lower thermal and chemical stability compared to zeolites due to their coordination bonds between metal nodes and organic linkers, which can degrade at elevated temperatures or in harsh chemical environments. Zeolites, composed of robust aluminosilicate frameworks, maintain structural integrity under high thermal conditions (up to 800degC) and resist acidic and basic media better than most MOFs. Advances in synthesizing water-stable, thermally resilient MOFs have narrowed this gap, but zeolites remain preferred for applications demanding extreme durability.
Gas Storage and Separation Performance
Metal-Organic Frameworks (MOFs) exhibit superior gas storage capacity compared to zeolites due to their higher surface area and tunable pore structures, enabling enhanced adsorption of gases like hydrogen and methane. Zeolites offer robust thermal stability and selectivity for gas separation, particularly in industrial applications like CO2 capture and hydrocarbon separation. MOFs outperform zeolites in customizable pore size and functionality, leading to improved selectivity and storage density for specific gas molecules.
Catalytic Applications: MOFs vs Zeolites
Metal-organic frameworks (MOFs) exhibit superior tunability and larger surface areas compared to zeolites, enhancing catalytic efficiency in complex reactions such as selective oxidation and CO2 conversion. Zeolites offer exceptional shape selectivity and thermal stability, making them ideal for hydrocarbon cracking and petrochemical processes. Advances in MOF design now enable integration of catalytic sites with tailored pore environments, bridging performance gaps with traditional zeolitic catalysts in industrial applications.
Environmental and Energy Applications
Metal-Organic Frameworks (MOFs) exhibit superior surface area and tunable pore structures compared to zeolites, enabling enhanced carbon capture and gas storage for environmental applications. Zeolites, with their robust thermal stability and established catalytic properties, remain essential in industrial-scale energy production and pollution control. Emerging MOFs show potential for improved photocatalysis and hydrogen storage, offering sustainable energy solutions beyond the capabilities of traditional zeolite frameworks.
Industrial Challenges and Scalability
Metal-Organic Frameworks (MOFs) face significant industrial challenges due to their complex synthesis processes and stability issues under harsh operational conditions, limiting large-scale production and application. Zeolites offer superior thermal and chemical stability with well-established scalable manufacturing, making them more practical for industrial use despite MOFs' higher tunability and porosity. Scaling MOFs requires advancements in cost-effective synthesis, reproducibility, and durability to compete with the proven scalability of zeolite catalysts in petrochemical and gas separation industries.
Future Prospects and Emerging Trends
Metal-organic frameworks (MOFs) exhibit exceptional tunability and high surface areas, positioning them as promising candidates for advanced gas storage, catalysis, and drug delivery applications. Zeolites, known for their thermal stability and well-defined microporous structures, continue to dominate traditional catalytic processes and ion-exchange technologies. Emerging trends highlight the integration of MOFs with zeolitic materials to synergistically enhance selectivity and stability, driving innovations in environmental remediation and energy storage sectors.
Porosity gradients
Metal-Organic Frameworks (MOFs) exhibit tunable porosity gradients with high surface area and adjustable pore sizes, surpassing traditional zeolites in selective molecular adsorption and catalysis efficiency.
Topological diversity
Metal-organic frameworks (MOFs) exhibit greater topological diversity than zeolites due to their tunable organic linkers and metal nodes, enabling a wider range of pore structures and functionalities.
Framework flexibility
Metal-organic frameworks (MOFs) exhibit superior framework flexibility compared to zeolites, enabling dynamic structural adjustments for improved adsorption and catalytic performance.
Gas uptake selectivity
Metal-Organic Frameworks (MOFs) exhibit higher gas uptake selectivity compared to zeolites due to their tunable pore structures and adjustable chemical functionalities.
Hydrothermal stability
Metal-Organic Frameworks (MOFs) generally exhibit lower hydrothermal stability compared to zeolites, which maintain structural integrity under high-temperature and high-moisture conditions due to their robust aluminosilicate frameworks.
Post-synthetic modification
Post-synthetic modification of MOFs allows precise tuning of their pore structure and chemical functionality, offering greater versatility than zeolites, which have more limited modification capabilities due to their rigid crystalline frameworks.
Cation exchange capacity
Metal-Organic Frameworks (MOFs) demonstrate significantly higher cation exchange capacities than zeolites due to their larger surface areas and tunable pore structures.
Metal–ligand coordination
Metal-ligand coordination in MOFs allows tunable pore structures and chemical functionalities, offering greater versatility than the rigid, aluminosilicate frameworks of zeolites.
Crystallographic disorder
Metal-Organic Frameworks (MOFs) exhibit higher crystallographic disorder compared to zeolites due to their flexible organic linkers and diverse coordination environments, impacting their structural stability and functional properties.
Guest molecule encapsulation
Metal-Organic Frameworks (MOFs) offer higher tunability and larger pore volumes than zeolites, enabling more efficient and selective encapsulation of diverse guest molecules for applications in catalysis, gas storage, and drug delivery.
MOFs vs Zeolites Infographic
 njnir.com
njnir.com