Thermal conductivity and electrical conductivity are critical properties in materials engineering, reflecting a material's ability to conduct heat and electric current, respectively. Metals typically exhibit high values for both properties due to free electrons facilitating simultaneous thermal and electrical transport. Understanding the interplay between these conductivities guides the design of materials for applications like thermoelectrics, where optimizing heat dissipation and electrical efficiency is essential.
Table of Comparison
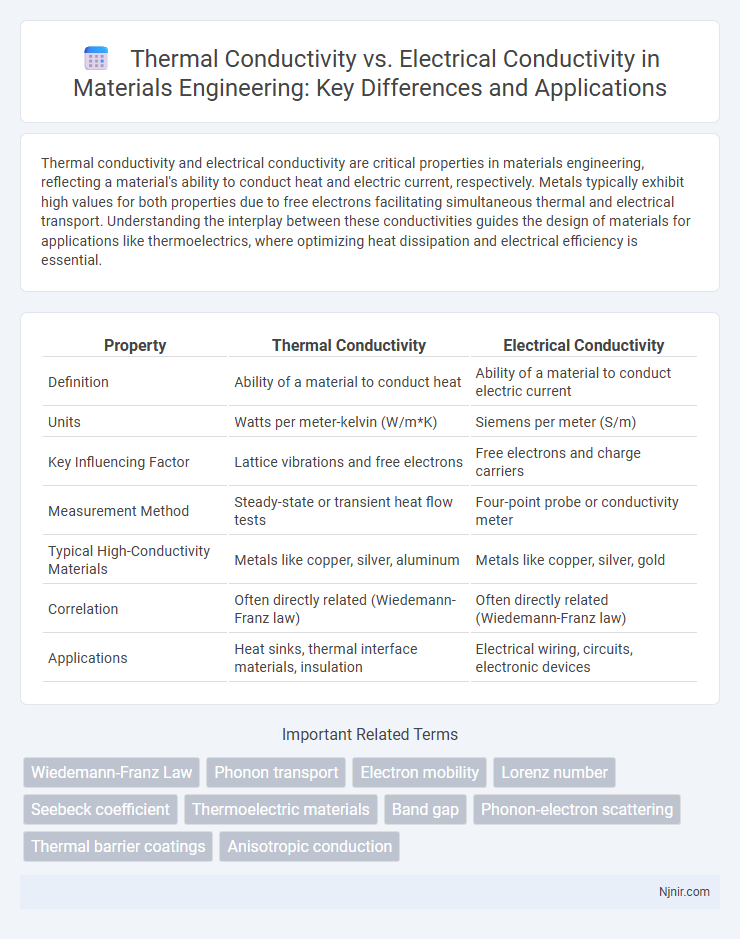
| Property | Thermal Conductivity | Electrical Conductivity |
|---|---|---|
| Definition | Ability of a material to conduct heat | Ability of a material to conduct electric current |
| Units | Watts per meter-kelvin (W/m*K) | Siemens per meter (S/m) |
| Key Influencing Factor | Lattice vibrations and free electrons | Free electrons and charge carriers |
| Measurement Method | Steady-state or transient heat flow tests | Four-point probe or conductivity meter |
| Typical High-Conductivity Materials | Metals like copper, silver, aluminum | Metals like copper, silver, gold |
| Correlation | Often directly related (Wiedemann-Franz law) | Often directly related (Wiedemann-Franz law) |
| Applications | Heat sinks, thermal interface materials, insulation | Electrical wiring, circuits, electronic devices |
Introduction to Thermal and Electrical Conductivity
Thermal conductivity measures a material's ability to transfer heat, typically expressed in watts per meter-kelvin (W/m*K), and is crucial in applications like heat sinks and insulation. Electrical conductivity quantifies how well a material can conduct an electric current, with units of siemens per meter (S/m), essential for designing electrical circuits and components. Both properties depend on the material's atomic structure and electron mobility, influencing performance in electronics, thermoelectrics, and energy systems.
Fundamental Concepts and Definitions
Thermal conductivity measures a material's ability to conduct heat, expressed in watts per meter-kelvin (W/m*K), while electrical conductivity quantifies its capacity to conduct electric current, given in siemens per meter (S/m). Unlike thermal conductivity, which depends on lattice vibrations (phonons) and free electron movement, electrical conductivity primarily arises from the density and mobility of charge carriers such as electrons. These fundamental properties are governed by material structure, temperature, and intrinsic bonding, influencing applications in thermoelectric devices and electrical wiring.
Mechanisms of Heat and Charge Transport
Thermal conductivity primarily depends on lattice vibrations (phonons) and free electron movement, where phonons dominate in insulators and free electrons in metals. Electrical conductivity is governed by the flow of free electrons or charge carriers responding to an electric field, with scattering mechanisms influencing both thermal and electrical transport. The Wiedemann-Franz law quantifies the relationship between thermal and electrical conductivities in metals, linking heat and charge transport through electron behavior.
Key Factors Affecting Thermal Conductivity
Thermal conductivity in materials is primarily influenced by factors such as atomic structure, bonding type, and lattice vibrations, where metals typically exhibit high thermal conductivity due to free electron movement. In contrast, electrical conductivity depends largely on the availability and mobility of charge carriers like electrons or holes, with metals again having high values due to their electron sea. Defects, impurities, temperature changes, and phonon scattering significantly affect thermal conductivity, often reducing it as these disruptions impede heat transfer through vibrations in the crystal lattice.
Determinants of Electrical Conductivity in Materials
Electrical conductivity in materials primarily depends on the availability and mobility of free charge carriers such as electrons or ions, with metals exhibiting high conductivity due to their free electron sea. Impurities, temperature, and lattice defects also significantly influence electrical conductivity by scattering charge carriers and impeding their flow. Unlike thermal conductivity, which is heavily influenced by lattice vibrations (phonons), electrical conductivity hinges on electron movement governed by the material's band structure and carrier concentration.
Comparative Analysis: Metals, Ceramics, and Polymers
Metals exhibit high thermal and electrical conductivity due to free electrons facilitating efficient energy transfer, with copper and silver leading in both parameters. Ceramics generally have low electrical conductivity because of their ionic or covalent bonding but can possess moderate to high thermal conductivity, as seen in aluminum nitride and silicon carbide. Polymers typically show low conductivity in both domains due to their molecular structure, though certain conductive polymers and composites demonstrate improved electrical properties while maintaining poor thermal conduction.
Role of Microstructure in Conductivity
The microstructure of a material, including grain size, phase distribution, and defect density, significantly influences both thermal conductivity and electrical conductivity by affecting electron and phonon scattering mechanisms. Fine-grained materials typically exhibit increased grain boundary scattering, reducing electrical conductivity but can also disrupt phonon transport, lowering thermal conductivity. Tailoring microstructural features such as crystallinity and impurity distribution enables optimization of the balance between electrical and thermal conductivities for applications in thermoelectrics and electronic devices.
Temperature Dependence of Conductivities
Thermal conductivity and electrical conductivity exhibit distinct temperature dependencies due to their physical mechanisms; thermal conductivity in metals typically decreases with rising temperature because of increased phonon scattering, while electrical conductivity decreases as temperature increases due to enhanced electron-phonon collisions. In semiconductors, thermal conductivity may vary nonlinearly with temperature, influenced by lattice vibrations and impurity scattering, whereas electrical conductivity generally increases with temperature due to greater carrier concentration. Understanding these dependencies is critical for optimizing materials in thermoelectric devices and electronic components operating across varying thermal environments.
Engineering Applications and Material Selection
Thermal conductivity and electrical conductivity critically influence material selection in engineering applications such as heat exchangers, electronic packaging, and power transmission. Materials like copper and aluminum are preferred for their high electrical and thermal conductivities, enhancing efficiency in electrical wiring and heat dissipation systems. Engineers balance these properties with mechanical strength and corrosion resistance to optimize performance in specific industrial environments.
Future Trends in Conductive Materials Research
Future trends in conductive materials research emphasize enhancing thermal conductivity while maintaining or improving electrical conductivity to optimize energy efficiency in electronics and renewable energy systems. Advanced materials such as graphene, carbon nanotubes, and novel polymers exhibit promising synergistic properties, enabling next-generation thermoelectric devices and flexible electronics. Continued innovation in nanoscale engineering and hybrid composites is critical for tailoring conductive pathways and achieving superior performance in diverse applications.
Wiedemann-Franz Law
The Wiedemann-Franz Law quantifies the direct proportionality between thermal conductivity and electrical conductivity in metals, governed by the Lorenz number.
Phonon transport
Phonon transport governs thermal conductivity in insulators and semiconductors by facilitating lattice vibrations, whereas electrical conductivity depends primarily on electron movement, resulting in distinct mechanisms for heat and charge transfer.
Electron mobility
Higher electron mobility enhances both thermal conductivity and electrical conductivity by facilitating efficient electron flow and energy transfer.
Lorenz number
The Lorenz number quantifies the ratio of thermal conductivity to electrical conductivity in metals, reflecting the proportional relationship defined by the Wiedemann-Franz law.
Seebeck coefficient
Materials with high electrical conductivity often exhibit low thermal conductivity, which enhances the Seebeck coefficient and improves thermoelectric efficiency.
Thermoelectric materials
Thermoelectric materials exhibit low thermal conductivity and high electrical conductivity to maximize the efficiency of heat-to-electricity conversion.
Band gap
Materials with a smaller band gap typically exhibit higher electrical conductivity but lower thermal conductivity due to increased carrier mobility and reduced phonon scattering.
Phonon-electron scattering
Phonon-electron scattering reduces thermal conductivity by disrupting lattice vibrations while simultaneously affecting electrical conductivity by scattering charge carriers in conductive materials.
Thermal barrier coatings
Thermal barrier coatings exhibit low thermal conductivity to protect components from heat while maintaining electrical conductivity tailored by specific ceramic and metallic layers for optimized performance.
Anisotropic conduction
Anisotropic conduction reveals that materials can exhibit significantly different thermal and electrical conductivity along different crystallographic directions, impacting the design of thermoelectric devices and anisotropic conductive films.
Thermal conductivity vs Electrical conductivity Infographic
 njnir.com
njnir.com