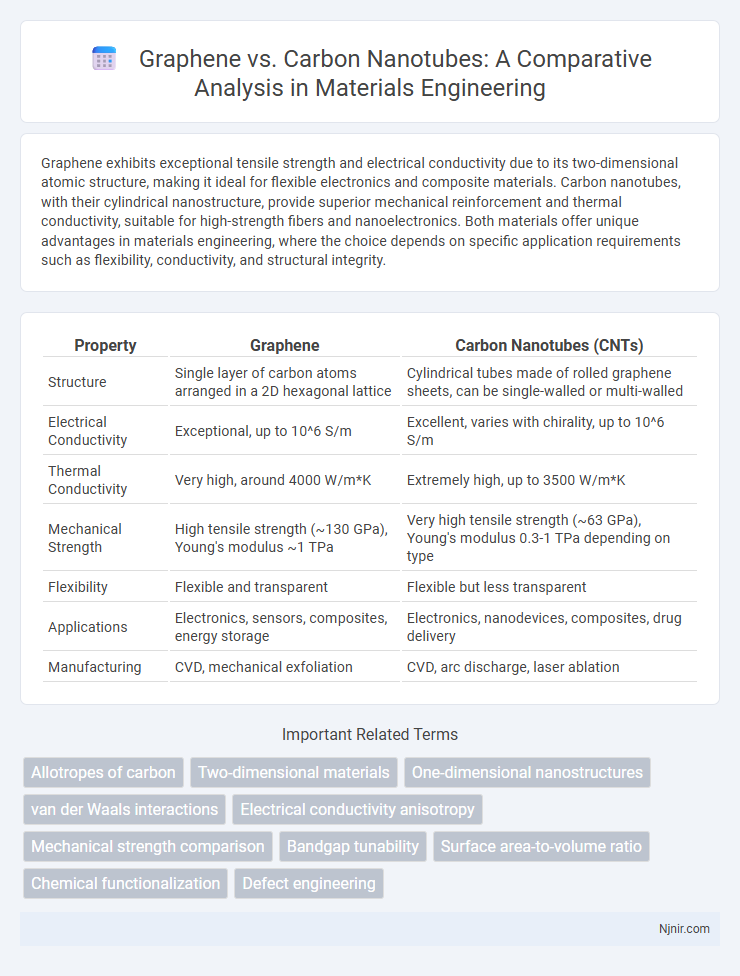
Graphene exhibits exceptional tensile strength and electrical conductivity due to its two-dimensional atomic structure, making it ideal for flexible electronics and composite materials. Carbon nanotubes, with their cylindrical nanostructure, provide superior mechanical reinforcement and thermal conductivity, suitable for high-strength fibers and nanoelectronics. Both materials offer unique advantages in materials engineering, where the choice depends on specific application requirements such as flexibility, conductivity, and structural integrity.
Table of Comparison
| Property | Graphene | Carbon Nanotubes (CNTs) |
|---|---|---|
| Structure | Single layer of carbon atoms arranged in a 2D hexagonal lattice | Cylindrical tubes made of rolled graphene sheets, can be single-walled or multi-walled |
| Electrical Conductivity | Exceptional, up to 10^6 S/m | Excellent, varies with chirality, up to 10^6 S/m |
| Thermal Conductivity | Very high, around 4000 W/m*K | Extremely high, up to 3500 W/m*K |
| Mechanical Strength | High tensile strength (~130 GPa), Young's modulus ~1 TPa | Very high tensile strength (~63 GPa), Young's modulus 0.3-1 TPa depending on type |
| Flexibility | Flexible and transparent | Flexible but less transparent |
| Applications | Electronics, sensors, composites, energy storage | Electronics, nanodevices, composites, drug delivery |
| Manufacturing | CVD, mechanical exfoliation | CVD, arc discharge, laser ablation |
Introduction to Graphene and Carbon Nanotubes
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, renowned for its exceptional electrical conductivity, mechanical strength, and flexibility. Carbon nanotubes consist of rolled-up sheets of graphene forming cylindrical structures with remarkable tensile strength and unique electronic properties determined by their chirality and diameter. Both materials exhibit extraordinary potential in nanoelectronics, energy storage, and composite materials due to their distinctive atomic structures and physical characteristics.
Structural Differences between Graphene and Carbon Nanotubes
Graphene consists of a single layer of carbon atoms arranged in a two-dimensional hexagonal lattice, providing exceptional strength and electrical conductivity. Carbon nanotubes are cylindrical structures formed by rolling graphene sheets into seamless tubes, which introduces unique mechanical properties and quantum effects. The key structural difference lies in graphene's flat planar form versus the tubular, one-dimensional structure of carbon nanotubes, influencing their respective electronic and mechanical behaviors.
Synthesis Methods for Graphene and Carbon Nanotubes
Graphene synthesis predominantly involves chemical vapor deposition (CVD), exfoliation, and epitaxial growth on silicon carbide substrates, enabling high-quality, large-area graphene films suitable for electronic applications. Carbon nanotubes (CNTs) are commonly synthesized using CVD, arc discharge, and laser ablation methods, with catalyst nanoparticles playing a crucial role in controlling tube diameter and chirality. The choice of synthesis method directly impacts the structural properties, purity, and scalability of graphene and CNTs for use in nanoelectronics, composites, and energy storage devices.
Mechanical Properties Comparison
Graphene exhibits exceptional tensile strength of approximately 130 GPa and Young's modulus near 1 TPa, making it one of the strongest known materials at the atomic scale. Carbon nanotubes (CNTs), with tensile strengths ranging from 30 to 100 GPa and Young's modulus values between 0.3 and 1 TPa, offer outstanding flexibility and resilience due to their cylindrical nanostructure. Both materials demonstrate superior mechanical properties, but graphene's two-dimensional lattice provides higher stiffness, whereas CNTs combine strength with enhanced toughness and impact resistance.
Electrical Conductivity and Electronic Applications
Graphene exhibits exceptional electrical conductivity due to its two-dimensional structure, enabling electron mobility exceeding 200,000 cm2/V*s, making it ideal for high-speed transistors and flexible electronics. Carbon nanotubes (CNTs), with their cylindrical nanostructure, also demonstrate remarkable conductivity but vary significantly between metallic and semiconducting types, influencing their usage in nanoscale interconnects and sensors. Both materials advance electronic applications, with graphene favoring transparent conductive films and CNTs excelling in field-effect transistors and nanoscale devices.
Thermal Properties and Heat Management
Graphene exhibits exceptional thermal conductivity up to 5000 W/mK, surpassing most materials including carbon nanotubes, which typically have thermal conductivities around 3000 W/mK. Its two-dimensional structure enables efficient heat dissipation, making graphene ideal for advanced heat management applications in electronics. Carbon nanotubes provide excellent directional heat transfer along their tubular axis but lack graphene's isotropic thermal performance, limiting their use in uniform heat spreading.
Chemical Stability and Reactivity
Graphene exhibits superior chemical stability due to its planar, sp2-bonded carbon lattice, which resists oxidation and chemical attack better than carbon nanotubes (CNTs). Carbon nanotubes, with their curved cylindrical structure and reactive edge sites, demonstrate higher reactivity, especially at defects and tube ends, making them more susceptible to functionalization and chemical modification. The difference in reactivity between graphene and CNTs fundamentally influences their performance in applications like sensors, catalysis, and composite materials.
Applications in Composite Materials
Graphene exhibits exceptional mechanical strength, electrical conductivity, and thermal stability, making it highly effective in enhancing the performance of composite materials used in aerospace, automotive, and sports equipment industries. Carbon nanotubes offer superior tensile strength and electrical conductivity, providing significant improvements in lightweight composites for structural reinforcement and electromagnetic interference shielding. Integrating graphene and carbon nanotubes into polymer matrices results in composites with enhanced durability, flexibility, and multifunctional properties essential for advanced engineering applications.
Environmental Impact and Sustainability
Graphene exhibits a lower environmental impact compared to carbon nanotubes due to its easier production methods and less energy-intensive processes, which result in reduced carbon emissions. The biodegradability and potential for recycling graphene-based materials also contribute to its sustainability, whereas carbon nanotubes often involve toxic catalysts and longer degradation times posing ecological risks. Sustainable development favors graphene as it supports scalable manufacturing with minimal hazardous waste, aligning well with green technology initiatives.
Future Trends in Graphene and Carbon Nanotube Research
Emerging studies reveal graphene's potential in flexible electronics and energy storage due to its exceptional conductivity and mechanical strength, while carbon nanotubes are gaining traction in nanoelectronics and composite materials for their high aspect ratio and tensile strength. Research increasingly focuses on hybrid structures combining graphene and carbon nanotubes to optimize electrical, thermal, and mechanical properties. Advancements in scalable production methods and functionalization techniques promise to unlock new applications in sensors, medical devices, and sustainable energy solutions.
Allotropes of carbon
Graphene and carbon nanotubes are distinct carbon allotropes characterized by their unique atomic structures--graphene is a two-dimensional single layer of carbon atoms arranged in a hexagonal lattice, while carbon nanotubes are cylindrical nanostructures formed by rolling graphene sheets into tubes with exceptional mechanical, electrical, and thermal properties.
Two-dimensional materials
Graphene, a two-dimensional material consisting of a single layer of carbon atoms arranged in a hexagonal lattice, offers superior electrical conductivity and mechanical strength compared to carbon nanotubes, which are essentially rolled-up graphene sheets forming one-dimensional cylindrical structures.
One-dimensional nanostructures
Carbon nanotubes are one-dimensional nanostructures with exceptional electrical conductivity and mechanical strength, while graphene is a two-dimensional single layer of carbon atoms with remarkable surface area and flexibility.
van der Waals interactions
Van der Waals interactions in graphene provide stronger interlayer adhesion compared to carbon nanotubes, enhancing its structural stability and electronic properties in layered materials.
Electrical conductivity anisotropy
Graphene exhibits isotropic electrical conductivity within its plane, whereas carbon nanotubes demonstrate pronounced electrical conductivity anisotropy due to their cylindrical structure affecting electron transport along axial and radial directions.
Mechanical strength comparison
Graphene exhibits exceptional mechanical strength with a tensile strength of approximately 130 GPa, surpassing carbon nanotubes, which typically have tensile strengths around 60-100 GPa depending on their structure and chirality.
Bandgap tunability
Graphene's zero bandgap limits its tunability compared to carbon nanotubes, whose intrinsic semiconducting properties enable adjustable bandgaps through diameter and chirality variations.
Surface area-to-volume ratio
Graphene exhibits a higher surface area-to-volume ratio of approximately 2630 m2/g compared to carbon nanotubes' 1315 m2/g, making it more effective for applications requiring maximal surface exposure.
Chemical functionalization
Chemical functionalization of graphene offers higher surface area and diverse bonding sites compared to carbon nanotubes, enabling enhanced reactivity and tailored electronic properties for advanced material applications.
Defect engineering
Defect engineering in graphene enhances its electronic, mechanical, and chemical properties more controllably compared to carbon nanotubes, enabling tailored applications in sensors, nanoelectronics, and energy storage.
Graphene vs Carbon nanotubes Infographic
 njnir.com
njnir.com