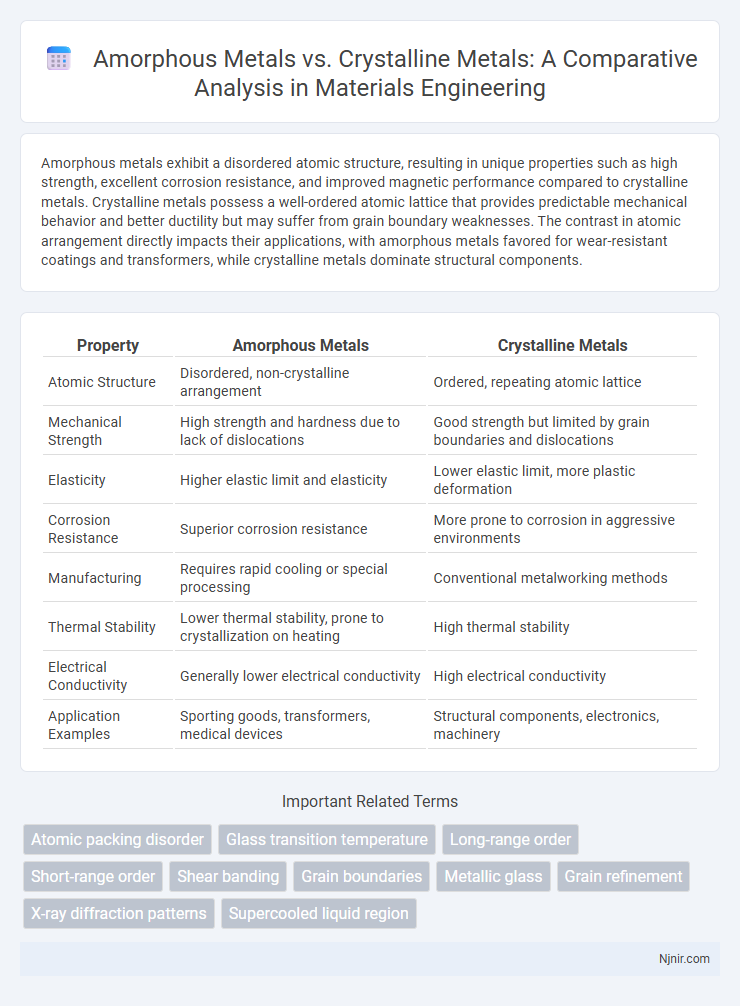
Amorphous metals exhibit a disordered atomic structure, resulting in unique properties such as high strength, excellent corrosion resistance, and improved magnetic performance compared to crystalline metals. Crystalline metals possess a well-ordered atomic lattice that provides predictable mechanical behavior and better ductility but may suffer from grain boundary weaknesses. The contrast in atomic arrangement directly impacts their applications, with amorphous metals favored for wear-resistant coatings and transformers, while crystalline metals dominate structural components.
Table of Comparison
| Property | Amorphous Metals | Crystalline Metals |
|---|---|---|
| Atomic Structure | Disordered, non-crystalline arrangement | Ordered, repeating atomic lattice |
| Mechanical Strength | High strength and hardness due to lack of dislocations | Good strength but limited by grain boundaries and dislocations |
| Elasticity | Higher elastic limit and elasticity | Lower elastic limit, more plastic deformation |
| Corrosion Resistance | Superior corrosion resistance | More prone to corrosion in aggressive environments |
| Manufacturing | Requires rapid cooling or special processing | Conventional metalworking methods |
| Thermal Stability | Lower thermal stability, prone to crystallization on heating | High thermal stability |
| Electrical Conductivity | Generally lower electrical conductivity | High electrical conductivity |
| Application Examples | Sporting goods, transformers, medical devices | Structural components, electronics, machinery |
Introduction to Amorphous and Crystalline Metals
Amorphous metals, also known as metallic glasses, feature a disordered atomic structure lacking the long-range periodicity found in crystalline metals, resulting in unique properties such as high strength and corrosion resistance. Crystalline metals possess a well-ordered, repeating atomic lattice, which contributes to their ductility and conductivity. Understanding the fundamental differences in atomic arrangement between amorphous and crystalline metals is essential for tailoring materials in advanced engineering applications.
Atomic Structure Differences
Amorphous metals exhibit a disordered atomic arrangement without a long-range repeating pattern, resulting in a non-crystalline structure. Crystalline metals have atoms organized in a highly ordered, repeating lattice that extends throughout the material. This fundamental difference in atomic structure influences mechanical properties, corrosion resistance, and magnetic behavior of both metal types.
Mechanical Properties Comparison
Amorphous metals exhibit superior strength and hardness compared to crystalline metals due to their disordered atomic structure, which prevents dislocation movement and enhances yield strength. Crystalline metals typically display higher ductility and toughness because their organized atomic lattice allows for plastic deformation through slip systems. The lack of grain boundaries in amorphous metals also results in improved wear resistance and corrosion resistance relative to their crystalline counterparts.
Electrical and Magnetic Characteristics
Amorphous metals exhibit significantly lower electrical conductivity than crystalline metals due to their lack of long-range atomic order, resulting in increased electron scattering. The magnetic properties of amorphous metals often include soft magnetic behavior with low coercivity and high permeability, making them ideal for transformer cores and magnetic shielding. Crystalline metals typically show higher electrical conductivity and more defined magnetic domains, leading to greater magnetic anisotropy and predictability in magnetic performance.
Corrosion and Wear Resistance
Amorphous metals exhibit superior corrosion resistance compared to crystalline metals due to their lack of grain boundaries, which reduces sites for corrosive attack and oxide formation. Their uniform atomic structure also enhances wear resistance by providing higher hardness and reduced friction compared to the crystalline counterparts. This makes amorphous metals ideal for applications requiring enhanced durability in harsh environments.
Manufacturing Processes and Challenges
Manufacturing amorphous metals involves rapid solidification techniques such as melt spinning and sputtering to prevent atomic crystallization, resulting in a non-ordered atomic structure. Crystalline metals, produced through traditional processes like casting, forging, and rolling, undergo controlled solidification allowing atoms to arrange into orderly lattices. Challenges in amorphous metal manufacturing include maintaining uniform cooling rates and avoiding crystallization, while crystalline metals face issues like grain boundary defects and anisotropy affecting mechanical properties.
Applications in Industry
Amorphous metals, also known as metallic glasses, are widely used in industries requiring high wear resistance and superior strength, such as in aerospace components, transformer cores, and sporting goods. Crystalline metals dominate applications demanding ductility and easy machinability, including automotive parts, construction materials, and electrical wiring. The unique atomic structure of amorphous metals enhances corrosion resistance and magnetic properties, making them ideal for precision instruments and specialized coatings.
Recent Advances in Metal Research
Recent advances in metal research reveal that amorphous metals, also known as metallic glasses, exhibit superior strength and corrosion resistance compared to their crystalline counterparts due to their disordered atomic structure. Innovations in additive manufacturing and rapid cooling techniques have enabled the production of larger amorphous metal components with enhanced mechanical properties and thermal stability. Studies using advanced microscopy and synchrotron radiation provide deeper insights into deformation mechanisms, enabling the design of novel alloys tailored for aerospace, biomedical, and electronic applications.
Environmental and Economic Impacts
Amorphous metals, also known as metallic glasses, generally require less energy for production compared to crystalline metals due to their rapid solidification process, reducing carbon emissions and lowering manufacturing costs. Their superior corrosion resistance and longer lifespan contribute to decreased material waste and fewer replacements, providing significant environmental and economic benefits. However, higher initial production costs and limited large-scale manufacturing capabilities currently restrict widespread adoption in various industries.
Future Prospects and Innovations
Amorphous metals, also known as metallic glasses, offer exceptional strength, corrosion resistance, and unique magnetic properties due to their non-crystalline atomic structure, positioning them as key materials for next-generation electronics, medical devices, and aerospace components. Advances in manufacturing techniques, such as rapid solidification and additive manufacturing, are enabling the scalable production of bulk amorphous metals, accelerating their integration into high-performance applications where traditional crystalline metals fall short. Research exploring hybrid composites combining amorphous and crystalline phases aims to optimize mechanical properties and expand functionality, driving innovations in lightweight armor, flexible electronics, and energy-efficient systems.
Atomic packing disorder
Amorphous metals exhibit atomic packing disorder characterized by a non-crystalline, irregular atomic arrangement, contrasting with crystalline metals that have a highly ordered, repeating atomic lattice.
Glass transition temperature
Amorphous metals exhibit a distinct glass transition temperature absent in crystalline metals, reflecting their non-crystalline atomic structure and influencing their thermal and mechanical properties.
Long-range order
Amorphous metals lack long-range atomic order, resulting in a non-crystalline structure, whereas crystalline metals exhibit a well-defined long-range periodic atomic arrangement.
Short-range order
Amorphous metals exhibit short-range atomic order lacking the long-range periodicity found in crystalline metals, resulting in unique mechanical and physical properties.
Shear banding
Amorphous metals exhibit more localized and intense shear banding compared to the more uniform and distributed shear deformation seen in crystalline metals due to their lack of grain boundaries and atomic order.
Grain boundaries
Amorphous metals lack grain boundaries due to their disordered atomic structure, resulting in improved corrosion resistance and mechanical strength compared to crystalline metals, which have defined grain boundaries that can act as sites for crack initiation and corrosion.
Metallic glass
Metallic glass, an amorphous metal characterized by a disordered atomic structure, exhibits superior strength, elasticity, and corrosion resistance compared to conventional crystalline metals with ordered lattices.
Grain refinement
Amorphous metals exhibit superior grain refinement compared to crystalline metals due to their non-crystalline atomic structure, resulting in enhanced strength and corrosion resistance.
X-ray diffraction patterns
Amorphous metals exhibit broad, diffuse X-ray diffraction patterns indicating a lack of long-range atomic order, whereas crystalline metals show sharp, well-defined diffraction peaks corresponding to their periodic atomic lattice.
Supercooled liquid region
Amorphous metals exhibit a significantly wider supercooled liquid region compared to crystalline metals, enabling enhanced plasticity and improved glass-forming ability.
Amorphous metals vs Crystalline metals Infographic
 njnir.com
njnir.com