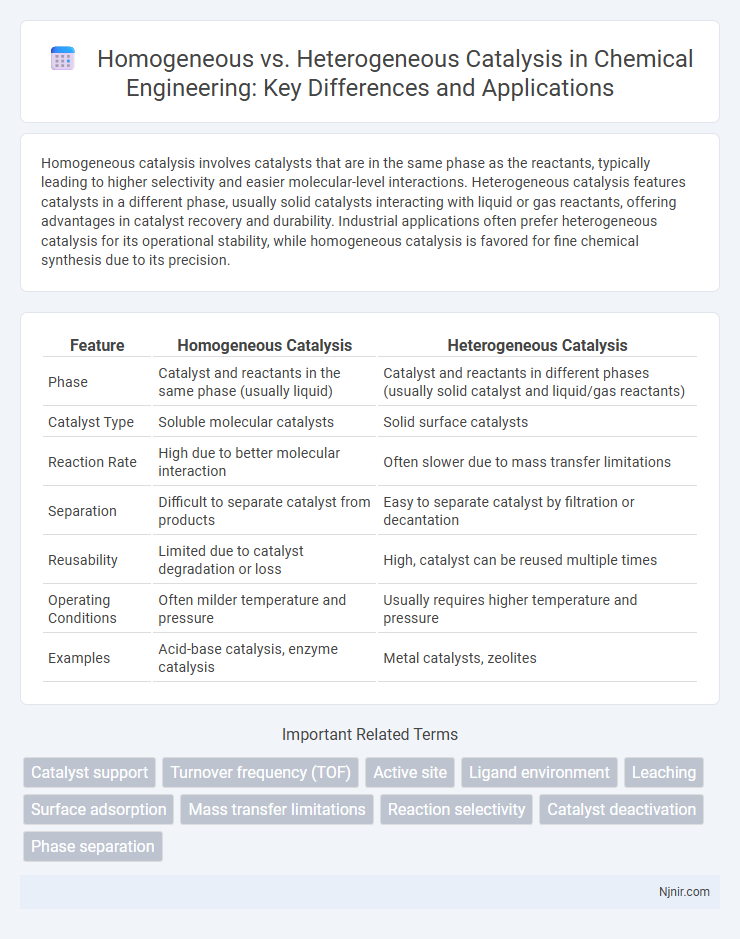
Homogeneous catalysis involves catalysts that are in the same phase as the reactants, typically leading to higher selectivity and easier molecular-level interactions. Heterogeneous catalysis features catalysts in a different phase, usually solid catalysts interacting with liquid or gas reactants, offering advantages in catalyst recovery and durability. Industrial applications often prefer heterogeneous catalysis for its operational stability, while homogeneous catalysis is favored for fine chemical synthesis due to its precision.
Table of Comparison
| Feature | Homogeneous Catalysis | Heterogeneous Catalysis |
|---|---|---|
| Phase | Catalyst and reactants in the same phase (usually liquid) | Catalyst and reactants in different phases (usually solid catalyst and liquid/gas reactants) |
| Catalyst Type | Soluble molecular catalysts | Solid surface catalysts |
| Reaction Rate | High due to better molecular interaction | Often slower due to mass transfer limitations |
| Separation | Difficult to separate catalyst from products | Easy to separate catalyst by filtration or decantation |
| Reusability | Limited due to catalyst degradation or loss | High, catalyst can be reused multiple times |
| Operating Conditions | Often milder temperature and pressure | Usually requires higher temperature and pressure |
| Examples | Acid-base catalysis, enzyme catalysis | Metal catalysts, zeolites |
Introduction to Catalysis in Chemical Engineering
Homogeneous catalysis occurs when the catalyst and reactants exist in the same phase, typically liquid, enabling uniform interaction and faster reaction kinetics essential in chemical engineering processes. Heterogeneous catalysis involves catalysts in a different phase, usually solid catalysts interacting with gaseous or liquid reactants, facilitating surface reactions critical for industrial applications like refining and emission control. Understanding these catalytic mechanisms optimizes reaction efficiency, selectivity, and scalability in chemical engineering design and operation.
Defining Homogeneous and Heterogeneous Catalysis
Homogeneous catalysis involves catalysts that exist in the same phase, typically liquid, as the reactants, enabling molecular-level interactions that facilitate reaction mechanisms. Heterogeneous catalysis features catalysts in a different phase, often solid catalysts interacting with gaseous or liquid reactants, promoting reactions at the surface through adsorption processes. The distinction hinges on phase uniformity, influencing catalyst recovery, reaction rates, and selectivity in industrial chemical processes.
Mechanisms of Homogeneous Catalysis
Homogeneous catalysis involves catalysts and reactants in the same phase, typically liquid, enabling molecular-level interactions that facilitate reaction pathways through stable intermediates and transition states. The mechanism often proceeds via coordination, oxidative addition, migratory insertion, and reductive elimination, allowing precise control over reaction specificity and rates. This contrasts with heterogeneous catalysis, where surface interactions dominate and catalytic sites are less uniform.
Mechanisms of Heterogeneous Catalysis
Heterogeneous catalysis involves the interaction of reactants with a solid catalyst surface where adsorption, surface reaction, and desorption occur, facilitating the transformation into products. The mechanism typically includes steps such as physical or chemical adsorption of reactants, surface diffusion to active sites, reaction on the catalyst surface, and subsequent desorption of products. Key factors influencing the efficiency include the catalyst's surface area, active site availability, and the nature of the adsorbate-catalyst bond.
Advantages of Homogeneous Catalysis
Homogeneous catalysis offers superior selectivity and uniform catalyst dispersion, resulting in enhanced reaction rates and product purity. The catalyst operates in the same phase as the reactants, enabling precise molecular interactions and easier kinetic control. This approach also allows for straightforward catalyst modification to tailor activity and selectivity for specific chemical transformations.
Advantages of Heterogeneous Catalysis
Heterogeneous catalysis offers significant advantages, including easy separation of catalyst from reaction products, which enhances catalyst recyclability and reduces contamination in the final product. Its solid catalysts provide high surface area-to-volume ratios, improving reaction rates and enabling operation under a wide range of conditions. Industrial processes benefit from heterogeneous catalysis through increased stability, durability, and the ability to tailor catalyst properties for specific reactions.
Challenges and Limitations of Both Catalytic Systems
Homogeneous catalysis often faces challenges such as difficult catalyst separation and recycling, limited thermal stability, and sensitivity to impurities, which can hinder industrial scalability and increase operational costs. Heterogeneous catalysis, while easier to separate and recycle, is limited by lower selectivity, possible catalyst deactivation due to sintering or poisoning, and mass transfer limitations affecting reaction rates. Both systems require optimization to balance activity, selectivity, and durability while addressing specific process conditions and economic feasibility.
Catalyst Recovery and Reusability
Homogeneous catalysis involves catalysts that are in the same phase as the reactants, typically liquid, which complicates catalyst recovery due to difficulties in separation, often requiring complex purification techniques. Heterogeneous catalysis uses solid catalysts with reactants in different phases, enabling easy separation and straightforward recovery through filtration or decantation, enhancing catalyst reusability. The solid nature of heterogeneous catalysts generally provides superior stability and longevity, making them more efficient for industrial applications that demand repeated use and cost-effectiveness.
Industrial Applications and Case Studies
Homogeneous catalysis, involving catalysts in the same phase as reactants, is widely used in fine chemical synthesis, such as hydroformylation in the production of aldehydes and pharmaceuticals, offering high selectivity and mild operating conditions. Heterogeneous catalysis, with solid catalysts acting on gaseous or liquid reactants, dominates large-scale industrial processes like ammonia synthesis via the Haber-Bosch process and catalytic cracking in petroleum refining, prized for catalyst recyclability and robustness. Case studies in the petrochemical industry demonstrate heterogeneous catalysts' efficiency in continuous production, whereas homogeneous catalysts excel in specialty chemical manufacturing where product purity and catalyst specificity are critical.
Future Trends in Catalysis Research
Future trends in catalysis research emphasize the development of hybrid catalytic systems that integrate the selectivity of homogeneous catalysis with the durability of heterogeneous catalysts. Advances in nanotechnology and machine learning-driven catalyst design are accelerating the discovery of novel active sites with enhanced efficiency and sustainability. Research increasingly targets renewable resource conversion and environmental applications, prioritizing catalysts that enable carbon-neutral chemical processes and energy storage technologies.
Catalyst support
Catalyst supports in homogeneous catalysis typically involve soluble ligands that stabilize metal centers, whereas heterogeneous catalysis relies on solid supports like oxides or carbons to disperse and anchor active metal sites for enhanced reaction efficiency.
Turnover frequency (TOF)
Heterogeneous catalysis typically exhibits lower turnover frequency (TOF) than homogeneous catalysis due to limited active site accessibility and mass transfer constraints.
Active site
Homogeneous catalysis features active sites uniformly dispersed within the reaction medium, enabling molecular-level interactions, while heterogeneous catalysis involves active sites located on solid surfaces, facilitating substrate adsorption and surface-mediated reactions.
Ligand environment
Homogeneous catalysis features a well-defined ligand environment that enables precise control of catalytic activity and selectivity, whereas heterogeneous catalysis involves less defined surface ligand interactions, leading to broader active site variability.
Leaching
Leaching in heterogeneous catalysis involves the undesired dissolution of active metal species into the reaction solution, reducing catalyst stability, whereas homogeneous catalysis inherently operates with fully soluble catalysts, eliminating leaching concerns.
Surface adsorption
Homogeneous catalysis occurs in a uniform phase with reactants and catalysts fully mixed, while heterogeneous catalysis relies on surface adsorption where reactants bind to the catalyst surface, enabling reaction at the interface.
Mass transfer limitations
Homogeneous catalysis involves catalysts dissolved in the same phase as reactants, minimizing mass transfer limitations, whereas heterogeneous catalysis faces significant mass transfer resistance due to phase boundaries between solid catalysts and reactants in different phases.
Reaction selectivity
Homogeneous catalysis offers higher reaction selectivity due to uniform active sites and better molecular-level interactions compared to heterogeneous catalysis, which often exhibits lower selectivity caused by varied surface sites.
Catalyst deactivation
Catalyst deactivation in homogeneous catalysis often results from ligand degradation or complex decomposition, while in heterogeneous catalysis it primarily occurs due to surface poisoning, sintering, or fouling.
Phase separation
Homogeneous catalysis involves catalysts and reactants in the same phase, allowing molecular-level interaction, while heterogeneous catalysis features catalysts in a different phase, facilitating easy separation and recycling through phase boundary processes.
Homogeneous catalysis vs Heterogeneous catalysis Infographic
 njnir.com
njnir.com