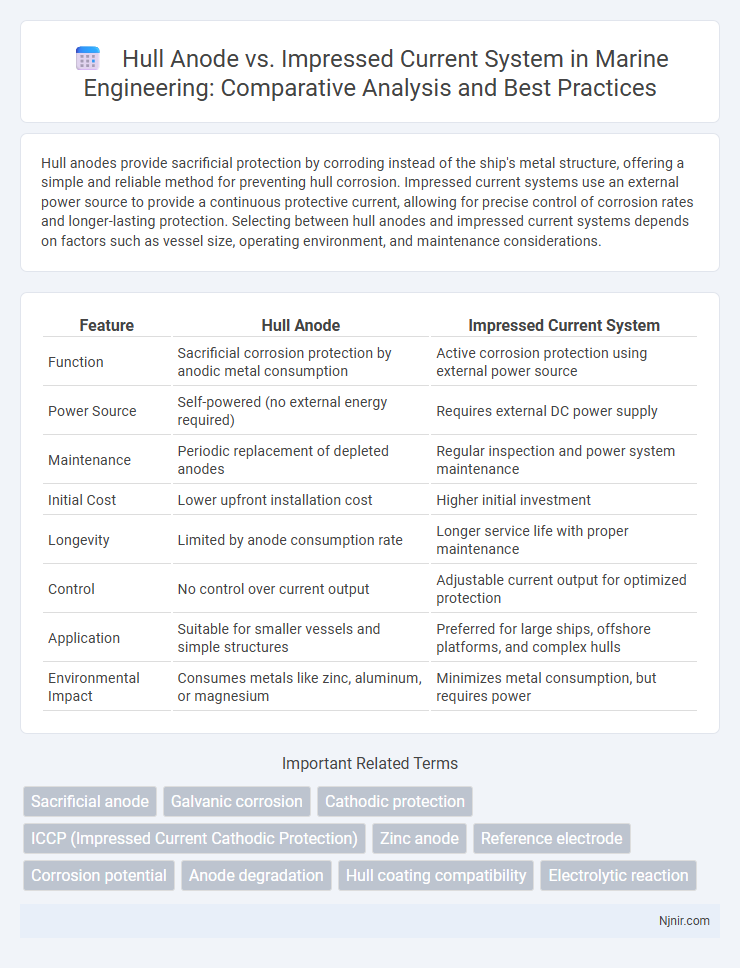
Hull anodes provide sacrificial protection by corroding instead of the ship's metal structure, offering a simple and reliable method for preventing hull corrosion. Impressed current systems use an external power source to provide a continuous protective current, allowing for precise control of corrosion rates and longer-lasting protection. Selecting between hull anodes and impressed current systems depends on factors such as vessel size, operating environment, and maintenance considerations.
Table of Comparison
| Feature | Hull Anode | Impressed Current System |
|---|---|---|
| Function | Sacrificial corrosion protection by anodic metal consumption | Active corrosion protection using external power source |
| Power Source | Self-powered (no external energy required) | Requires external DC power supply |
| Maintenance | Periodic replacement of depleted anodes | Regular inspection and power system maintenance |
| Initial Cost | Lower upfront installation cost | Higher initial investment |
| Longevity | Limited by anode consumption rate | Longer service life with proper maintenance |
| Control | No control over current output | Adjustable current output for optimized protection |
| Application | Suitable for smaller vessels and simple structures | Preferred for large ships, offshore platforms, and complex hulls |
| Environmental Impact | Consumes metals like zinc, aluminum, or magnesium | Minimizes metal consumption, but requires power |
Introduction to Hull Protection in Marine Engineering
Hull anode systems use sacrificial metals like zinc or aluminum to protect ship hulls through galvanic corrosion, offering a passive, maintenance-friendly solution for small to medium-sized vessels. Impressed current systems employ an external power source to provide a controlled electric current, enabling effective protection for larger ships with complex hull geometries or harsh marine environments. Both methods are critical in marine engineering to prevent corrosion, extend hull lifespan, and reduce maintenance costs, with the choice depending on vessel size, operating conditions, and cost considerations.
Understanding Hull Anodes: Types and Functions
Hull anodes, primarily made from zinc, aluminum, or magnesium, serve as sacrificial anodes to protect ship hulls from galvanic corrosion by corroding in place of the metal structure. These anodes come in various types, including ribbon, block, and extruded forms, each designed to suit specific hull shapes and operational environments for optimal protection. Their function is to provide a controlled source of electric current that prevents the hull metal from oxidizing, ensuring structural integrity and extending vessel lifespan.
Overview of Impressed Current Cathodic Protection (ICCP)
Impressed Current Cathodic Protection (ICCP) systems use an external power source to apply a continuous electrical current, effectively preventing metal corrosion on ship hulls and underwater structures. This system offers superior control over protection levels compared to sacrificial hull anodes, enabling adjustment to varying environmental conditions such as seawater resistivity and temperature. ICCP is widely preferred for large vessels and offshore installations due to its long lifespan, reduced maintenance, and enhanced efficiency in minimizing electrochemical deterioration.
Hull Anode Systems: Advantages and Limitations
Hull anode systems offer a reliable and cost-effective solution for cathodic protection, with the primary advantage of simplicity in design and low maintenance requirements. These systems typically employ sacrificial anodes bonded directly to the hull, providing consistent corrosion protection without the need for external power sources. However, limitations include their reduced effectiveness in large vessels or harsh marine environments and the need for periodic replacement of consumed anodes, which can increase operational downtime.
Impressed Current Systems: Advantages and Limitations
Impressed current systems offer precise control over corrosion protection by supplying a continuous, regulated DC current to the hull, extending the lifespan of marine vessels significantly. These systems require an external power source, which increases operational costs but allows for adjustable protection levels suitable for varying environmental conditions. Limitations include the potential for overprotection leading to coating damage and the need for regular maintenance to ensure system reliability and efficiency.
Comparative Analysis: Hull Anodes vs ICCP Systems
Hull anodes offer a passive protection method using sacrificial metals like zinc or aluminum, which corrodes to protect the ship's hull, while impressed current cathodic protection (ICCP) systems actively supply a controlled current to prevent corrosion. Hull anodes require regular replacement due to consumption, whereas ICCP systems have longer service lives and allow precise adjustment for optimal protection across varying water conditions. ICCP systems generally involve higher upfront costs but reduce maintenance efforts and provide more uniform protection compared to the straightforward installation and lower initial expense of hull anodes.
Installation and Maintenance Requirements
Hull anode systems involve attaching sacrificial anodes directly to the ship's hull, requiring routine visual inspections and periodic replacement due to gradual consumption. Impressed current cathodic protection (ICCP) systems utilize an external power source to deliver current through inert anodes, demanding more complex installation with electrical wiring and regular monitoring of power output and anode condition. Maintenance for ICCP systems includes checking transformer-rectifiers and controller settings, while hull anodes primarily need monitoring for depletion and securing to prevent detachment.
Cost Considerations: Initial and Long-Term Expenses
Hull anode systems typically involve higher initial costs due to the price of sacrificial anodes and installation labor but offer lower ongoing maintenance expenses since the anodes self-sacrifice over time. Impressed current cathodic protection (ICCP) systems require a significant upfront investment in power supplies, control units, and wiring, with long-term energy consumption and periodic component replacement adding to operating costs. Evaluating total cost of ownership requires factoring both the capital expenditure and operational expenses associated with each system's durability and maintenance requirements.
Suitability for Vessel Types and Operational Conditions
Hull anodes provide effective cathodic protection for small to medium-sized vessels with minimal maintenance requirements, making them suitable for recreational boats and fishing vessels operating in calm or moderate waters. Impressed current systems are ideal for larger commercial ships and offshore structures exposed to aggressive marine environments, as they offer adjustable protection levels tailored to varying water conditions and hull materials. The operational flexibility and enhanced control of impressed current systems ensure optimal corrosion prevention under dynamic and harsh operational scenarios.
Future Trends in Marine Hull Protection Systems
Future trends in marine hull protection emphasize the integration of advanced impressed current cathodic protection (ICCP) systems enhanced by smart sensors and IoT connectivity to enable real-time corrosion monitoring and adaptive current control. Hull anodes, traditionally favored for simplicity and reliability, are being gradually supplemented or replaced by ICCP solutions due to their efficiency in protecting larger vessels and complex hull geometries. Innovations in materials science, such as titanium mesh anodes and nanocoatings, further improve the longevity and environmental compatibility of both hull anode and impressed current systems.
Sacrificial anode
Sacrificial anodes, commonly made of zinc or magnesium, provide passive corrosion protection by corroding instead of the ship's hull, while impressed current systems use external power sources to actively prevent corrosion.
Galvanic corrosion
Hull anodes provide passive cathodic protection against galvanic corrosion by sacrificing themselves, while impressed current systems actively control corrosion using an external power source to maintain a protective current on the hull.
Cathodic protection
Hull anodes provide passive cathodic protection by sacrificially corroding to protect the ship's structure, while impressed current systems actively supply electrical current to prevent corrosion more effectively on larger vessels.
ICCP (Impressed Current Cathodic Protection)
ICCP (Impressed Current Cathodic Protection) uses an external power source to provide a continuous protective current to ship hull anodes, effectively preventing corrosion more reliably than traditional sacrificial anode systems.
Zinc anode
Zinc anodes in hull protection provide cost-effective, passive corrosion prevention through sacrificial oxidation, whereas impressed current systems actively control corrosion with adjustable electrical currents and higher installation costs.
Reference electrode
Reference electrodes in hull anode systems provide stable voltage measurements crucial for maintenance, while impressed current systems rely on reference electrodes for precise control of current output and optimized corrosion protection.
Corrosion potential
Hull anodes provide consistent low corrosion potential protection through sacrificial metal oxidation, while impressed current systems achieve adjustable and higher corrosion potential control using external power sources.
Anode degradation
Hull anodes in impressed current systems experience faster degradation due to localized electrochemical wear compared to more uniformly distributed consumption in sacrificial anode systems.
Hull coating compatibility
Hull anodes provide passive corrosion protection compatible with most hull coatings, while impressed current systems require careful compatibility assessment to prevent coating degradation and ensure effective protection.
Electrolytic reaction
Hull anode systems rely on passive electrolytic reactions producing sacrificial metal dissolution, while impressed current systems use externally supplied current to control and optimize the cathodic protection rate and minimize metal loss.
hull anode vs impressed current system Infographic
 njnir.com
njnir.com