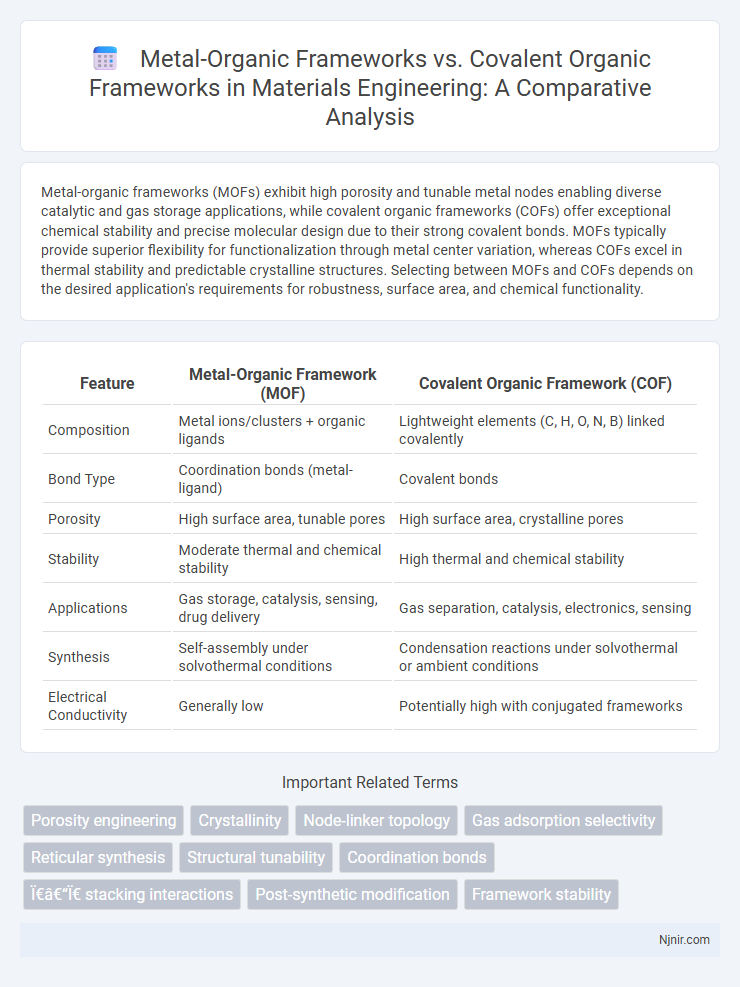
Metal-organic frameworks (MOFs) exhibit high porosity and tunable metal nodes enabling diverse catalytic and gas storage applications, while covalent organic frameworks (COFs) offer exceptional chemical stability and precise molecular design due to their strong covalent bonds. MOFs typically provide superior flexibility for functionalization through metal center variation, whereas COFs excel in thermal stability and predictable crystalline structures. Selecting between MOFs and COFs depends on the desired application's requirements for robustness, surface area, and chemical functionality.
Table of Comparison
| Feature | Metal-Organic Framework (MOF) | Covalent Organic Framework (COF) |
|---|---|---|
| Composition | Metal ions/clusters + organic ligands | Lightweight elements (C, H, O, N, B) linked covalently |
| Bond Type | Coordination bonds (metal-ligand) | Covalent bonds |
| Porosity | High surface area, tunable pores | High surface area, crystalline pores |
| Stability | Moderate thermal and chemical stability | High thermal and chemical stability |
| Applications | Gas storage, catalysis, sensing, drug delivery | Gas separation, catalysis, electronics, sensing |
| Synthesis | Self-assembly under solvothermal conditions | Condensation reactions under solvothermal or ambient conditions |
| Electrical Conductivity | Generally low | Potentially high with conjugated frameworks |
Introduction to Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs)
Metal-Organic Frameworks (MOFs) are crystalline materials composed of metal ions coordinated to organic ligands, forming porous structures with high surface area ideal for gas storage, separation, and catalysis. Covalent Organic Frameworks (COFs) consist solely of light elements linked by strong covalent bonds, creating stable, highly ordered porous networks suitable for applications in drug delivery, sensing, and energy storage. Both MOFs and COFs exhibit tunable porosity and chemical functionality, but MOFs often provide greater structural diversity through metal centers while COFs offer enhanced chemical stability due to covalent bonding.
Structural Differences Between MOFs and COFs
Metal-organic frameworks (MOFs) consist of metal ions or clusters coordinated to organic ligands, forming highly porous crystalline structures, while covalent organic frameworks (COFs) are entirely composed of light elements such as carbon, hydrogen, nitrogen, and oxygen linked by strong covalent bonds in two- or three-dimensional networks. MOFs exhibit coordination bonds between metal nodes and organic linkers, resulting in diverse topologies and tunable pore sizes, whereas COFs rely on robust covalent bonds creating more rigid and chemically stable frameworks with precise atomic arrangements. The structural differences lead to variations in thermal stability, chemical robustness, and potential applications in gas storage, catalysis, and molecular separation.
Synthesis Strategies for MOFs and COFs
Metal-organic frameworks (MOFs) are synthesized through coordination-driven self-assembly of metal ions or clusters with organic ligands, often employing solvothermal or hydrothermal methods to achieve crystalline structures. Covalent organic frameworks (COFs) are created by covalent bond formation between organic monomers, typically using dynamic covalent chemistry techniques such as Schiff-base condensation or boronic acid dehydration under reversible reaction conditions. Both MOFs and COFs synthesis strategies prioritize precise control over pore size, topology, and crystallinity to optimize their applications in gas storage, catalysis, and sensing.
Porosity and Surface Area Comparison
Metal-organic frameworks (MOFs) typically exhibit higher surface areas, often exceeding 7,000 m2/g, due to their metal nodes connected by organic linkers forming highly porous crystalline structures. Covalent organic frameworks (COFs), while generally more chemically stable, possess surface areas ranging from 500 to 2,500 m2/g, resulting from strong covalent bonds in their extended networks. MOFs offer superior porosity and tunable pore sizes, making them more versatile for gas storage and separation applications compared to COFs.
Thermal and Chemical Stability Analysis
Metal-organic frameworks (MOFs) generally exhibit moderate thermal stability, typically decomposing between 200degC and 400degC, depending on their metal clusters and organic linkers, whereas covalent organic frameworks (COFs) often demonstrate higher thermal stability, with decomposition temperatures commonly exceeding 400degC due to their strong covalent bonds. Chemical stability in MOFs varies widely based on metal-ligand coordination, with many MOFs susceptible to hydrolysis in humid or acidic environments, while COFs offer superior chemical resistance because of their robust covalent linkages, which resist degradation under acidic, basic, and oxidative conditions. Comparative thermal and chemical stability assessments highlight COFs as more suitable for applications requiring harsh environmental resilience, whereas MOFs are preferred for tailored porosity and functionality despite their relatively lower stability.
Gas Storage and Separation Capabilities
Metal-organic frameworks (MOFs) exhibit exceptional gas storage capabilities due to their high surface area and tunable pore sizes, enabling efficient adsorption of gases such as hydrogen, methane, and carbon dioxide. Covalent organic frameworks (COFs) offer robust chemical stability and precise structural regularity, which enhance selective gas separation and facilitate gas diffusion through ordered pore channels. Both materials show promise for gas storage and separation, but MOFs generally provide higher adsorption capacities, while COFs excel in chemical resistance and selectivity.
Applications in Catalysis: MOFs vs COFs
Metal-organic frameworks (MOFs) exhibit exceptional catalytic properties due to their high surface area, tunable pore sizes, and diverse metal centers, enabling effective catalysis in reactions such as CO2 reduction, hydrocarbon oxidation, and photocatalysis. Covalent organic frameworks (COFs) offer superior structural stability and enhanced electronic properties, making them ideal for catalysis involving organic transformations, electrocatalysis, and photocatalytic hydrogen evolution. MOFs generally outperform COFs in metal-centered catalysis, whereas COFs provide advantages in metal-free and semiconductor-based catalytic applications.
Environmental Remediation Potential
Metal-organic frameworks (MOFs) exhibit high surface area and tunable pore structures, enabling effective adsorption and catalytic degradation of pollutants in water and air, making them promising for environmental remediation. Covalent organic frameworks (COFs) offer exceptional chemical stability and tailored functionalities that enhance selective capture and photocatalytic breakdown of contaminants under mild conditions. Both frameworks demonstrate significant potential in removing heavy metals, organic dyes, and emerging pollutants, with MOFs generally providing higher adsorption capacities and COFs excelling in structural robustness.
Limitations and Challenges in MOFs and COFs
Metal-organic frameworks (MOFs) face challenges including limited thermal and chemical stability in harsh environments and potential framework collapse due to weak metal-ligand coordination bonds. Covalent organic frameworks (COFs) struggle with lower crystallinity and less established synthetic protocols, hindering large-scale production and structural predictability. Both MOFs and COFs encounter difficulties in achieving high conductivity and mechanical robustness necessary for advanced applications.
Future Perspectives in MOF and COF Research
Metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) represent innovative materials with vast potential in gas storage, catalysis, and drug delivery. The future of MOF research is expected to focus on enhancing stability, scalability, and functional diversification through advanced synthesis and computational design. COF research is poised to explore novel linkages and topologies to improve porosity, chemical robustness, and electronic properties for applications in energy storage and environmental remediation.
Porosity engineering
Metal-organic frameworks (MOFs) offer tunable porosity through variable metal nodes and organic linkers, while covalent organic frameworks (COFs) achieve precise porosity engineering via strong covalent bonds and customizable organic building blocks.
Crystallinity
Metal-organic frameworks (MOFs) typically exhibit higher crystallinity due to metal-ligand coordination bonds, whereas covalent organic frameworks (COFs) often show variable crystallinity influenced by covalent bond formation and synthesis conditions.
Node-linker topology
Metal-organic frameworks feature metal ion nodes connected by organic linkers creating versatile coordination geometries, while covalent organic frameworks consist of fully covalent bonding between organic nodes and linkers forming robust, crystalline, and porous networks.
Gas adsorption selectivity
Metal-organic frameworks exhibit higher gas adsorption selectivity due to their tunable metal nodes and porous structures compared to covalent organic frameworks, which have more rigid, purely organic backbones.
Reticular synthesis
Reticular synthesis enables the precise design of Metal-Organic Frameworks (MOFs) using metal nodes and organic linkers, while Covalent Organic Frameworks (COFs) rely on strong covalent bonds between organic building blocks to form highly crystalline, porous structures.
Structural tunability
Metal-organic frameworks (MOFs) offer greater structural tunability through diverse metal nodes and organic linkers compared to covalent organic frameworks (COFs), which rely solely on covalent bonds for modular design.
Coordination bonds
Metal-organic frameworks utilize coordination bonds between metal ions and organic ligands to create porous crystalline structures, whereas covalent organic frameworks rely on strong covalent bonds without metal centers, resulting in different stability and functionality profiles.
π–π stacking interactions
Metal-organic frameworks exhibit enhanced p-p stacking interactions due to metal-coordinated linkers, whereas covalent organic frameworks rely on strong covalent bonds, resulting in more stable but less flexible p-p stacking arrangements.
Post-synthetic modification
Post-synthetic modification of Metal-organic frameworks (MOFs) enables enhanced chemical functionality through metal node exchange and linker substitution, while Covalent organic frameworks (COFs) primarily rely on covalent bond transformations within their organic linkers to achieve tunable porosity and chemical stability.
Framework stability
Metal-organic frameworks exhibit high porosity but often lower chemical stability compared to covalent organic frameworks, which provide superior framework stability due to strong covalent bonds.
Metal-organic framework vs Covalent organic framework Infographic
 njnir.com
njnir.com