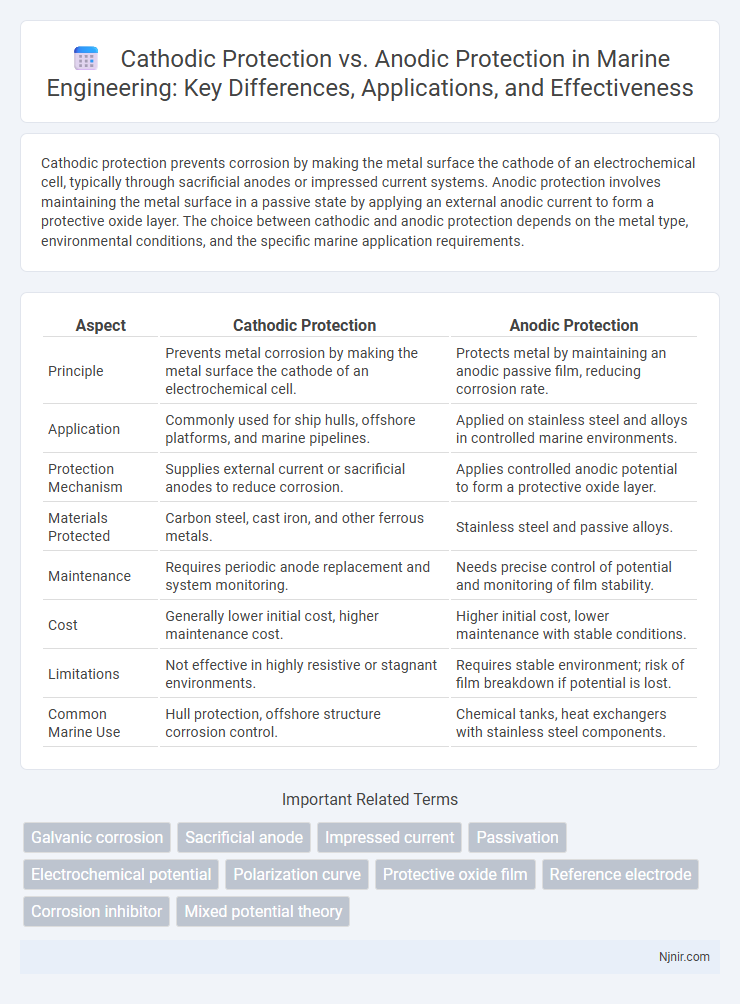
Cathodic protection prevents corrosion by making the metal surface the cathode of an electrochemical cell, typically through sacrificial anodes or impressed current systems. Anodic protection involves maintaining the metal surface in a passive state by applying an external anodic current to form a protective oxide layer. The choice between cathodic and anodic protection depends on the metal type, environmental conditions, and the specific marine application requirements.
Table of Comparison
| Aspect | Cathodic Protection | Anodic Protection |
|---|---|---|
| Principle | Prevents metal corrosion by making the metal surface the cathode of an electrochemical cell. | Protects metal by maintaining an anodic passive film, reducing corrosion rate. |
| Application | Commonly used for ship hulls, offshore platforms, and marine pipelines. | Applied on stainless steel and alloys in controlled marine environments. |
| Protection Mechanism | Supplies external current or sacrificial anodes to reduce corrosion. | Applies controlled anodic potential to form a protective oxide layer. |
| Materials Protected | Carbon steel, cast iron, and other ferrous metals. | Stainless steel and passive alloys. |
| Maintenance | Requires periodic anode replacement and system monitoring. | Needs precise control of potential and monitoring of film stability. |
| Cost | Generally lower initial cost, higher maintenance cost. | Higher initial cost, lower maintenance with stable conditions. |
| Limitations | Not effective in highly resistive or stagnant environments. | Requires stable environment; risk of film breakdown if potential is lost. |
| Common Marine Use | Hull protection, offshore structure corrosion control. | Chemical tanks, heat exchangers with stainless steel components. |
Introduction to Corrosion in Marine Engineering
Cathodic protection and anodic protection are essential corrosion control methods in marine engineering, targeting the electrochemical reactions causing metal deterioration in seawater environments. Cathodic protection prevents corrosion by applying a sacrificial anode or impressed current to make the protected metal a cathode, whereas anodic protection maintains the metal surface in a passive oxide film state by controlling the applied potential. Understanding these techniques is critical for extending the lifespan of marine structures like ship hulls, offshore platforms, and pipelines exposed to aggressive chloride ions.
Overview of Cathodic Protection
Cathodic protection is a corrosion control technique that protects metal surfaces by making them the cathode of an electrochemical cell, effectively preventing oxidation. It is widely used in pipelines, storage tanks, and marine structures to extend the lifespan of metallic infrastructure by minimizing corrosion rates. This method involves the use of sacrificial anodes or impressed current systems, where the metal surface is maintained at a lower electrical potential than the surrounding environment.
Principles of Anodic Protection
Anodic protection operates by maintaining the metal surface at a controlled positive potential within the passive region, thus forming a stable oxide film that significantly reduces corrosion rates. This method is particularly effective for metals like stainless steel in aggressive environments such as concentrated acids. The principle involves allowing a protective passive layer to form and remain intact, preventing metal dissolution by shifting the electrode potential into a region where passivation dominates.
Mechanisms of Corrosion Prevention
Cathodic protection prevents corrosion by supplying electrons to the metal surface, converting it into a cathode and thereby reducing the metal's oxidation rate. Anodic protection controls corrosion by maintaining the metal in a passive state through the formation of a stable oxide film on the anode surface, which acts as a barrier to further oxidation. Both mechanisms inhibit metal dissolution but differ fundamentally in electrochemical approach: cathodic protection suppresses anodic reactions through external current, while anodic protection stabilizes the metal surface by promoting passivation.
Comparative Analysis: Cathodic vs Anodic Protection
Cathodic protection prevents metal corrosion by supplying electrons, converting the metal surface into a cathode, while anodic protection controls corrosion by maintaining the metal in a passive state through controlled anodic current. Cathodic protection is commonly applied to pipelines, tanks, and marine structures, effectively mitigating uniform corrosion, whereas anodic protection is ideal for highly corrosive environments like acid storage tanks, offering superior resistance against localized corrosion. The choice between cathodic and anodic protection depends on factors such as metal type, environment, and corrosion mechanism, with cathodic systems typically being simpler and more cost-effective for general use, while anodic protection requires precise current control and monitoring for optimal performance.
Applications in Marine Structures
Cathodic protection is widely used in marine structures such as ship hulls, offshore platforms, and underwater pipelines to prevent corrosion by converting the metal surface into a cathode through sacrificial anodes or impressed current systems. Anodic protection is less common but applied in specific marine environments with aggressive conditions, like storage tanks or submerged steel structures exposed to oxidizing environments, where it maintains a passive oxide film on the metal surface. Both methods significantly extend the lifespan of marine assets by mitigating corrosion, but cathodic protection remains the preferred choice due to its versatility and cost-effectiveness in diverse seawater environments.
Installation and Maintenance Requirements
Cathodic protection installation typically involves attaching sacrificial anodes or impressed current systems to the metal structure, requiring regular inspection and replacement of anodes to ensure ongoing effectiveness. Anodic protection requires precise control of the metal's electrochemical potential through an external power source, necessitating sophisticated monitoring equipment and periodic calibration to maintain optimal protective conditions. Maintenance of cathodic protection is generally less complex but more frequent, while anodic protection demands higher initial setup expertise and ongoing specialized technical oversight.
Cost Considerations and Efficiency
Cathodic protection typically involves lower initial installation costs and simpler maintenance compared to anodic protection, making it more cost-effective for many large-scale infrastructure projects. Anodic protection offers higher efficiency in preventing corrosion for specific metals like stainless steel, but requires more complex control systems and higher operational expenses. The choice between these methods depends on balancing the long-term efficiency gains of anodic protection against the upfront cost savings and proven reliability of cathodic protection.
Environmental Impacts and Compliance
Cathodic protection reduces corrosion by making the metal surface a cathode, minimizing the release of harmful metal ions into the environment and ensuring compliance with environmental regulations for infrastructure longevity. Anodic protection forms a stable oxide layer on the metal, decreasing corrosion rates while potentially generating by-products requiring proper management to meet environmental standards. Both methods support regulatory compliance by extending asset life and reducing environmental contamination risks in industries such as oil, gas, and marine applications.
Future Trends in Corrosion Protection Technologies
Future trends in corrosion protection technologies emphasize advanced cathodic protection methods utilizing smart sensors and IoT integration for real-time monitoring and automatic adjustment of protective current. Anodic protection research is shifting towards nanomaterial coatings and electrochemical modulation to enhance passivation layers on highly reactive metals. Emerging hybrid systems combining cathodic and anodic strategies aim to maximize efficiency and durability in extreme industrial environments.
Galvanic corrosion
Cathodic protection prevents galvanic corrosion by making the metal surface the cathode through sacrificial anodes, whereas anodic protection controls corrosion by maintaining a protective oxide film on the metal surface acting as the anode.
Sacrificial anode
Sacrificial anode cathodic protection prevents corrosion by using a more reactive metal to corrode instead of the protected structure, unlike anodic protection which controls corrosion by maintaining the metal at a passive state through controlled anodic polarization.
Impressed current
Impressed current cathodic protection uses an external power source to provide a continuous protective current, whereas anodic protection involves maintaining a passive oxide film on the metal surface by controlling the potential to prevent corrosion.
Passivation
Cathodic protection prevents corrosion by supplying electrons to metal surfaces, while anodic protection creates a stable passive oxide layer through controlled anodic polarization, effectively utilizing passivation to inhibit metal dissolution.
Electrochemical potential
Cathodic protection lowers the metal's electrochemical potential to prevent corrosion by making it the cathode, while anodic protection raises the potential to form a passive oxide layer that inhibits further oxidation.
Polarization curve
Cathodic protection shifts the polarization curve to more negative potentials reducing corrosion rates by suppressing anodic reactions, while anodic protection moves the curve to a passive region at higher potentials, forming a protective oxide layer that minimizes metal dissolution.
Protective oxide film
Cathodic protection prevents corrosion by generating a protective oxide film through cathodic polarization that reduces metal oxidation, whereas anodic protection creates a stable, passive oxide film by promoting controlled anodic polarization to inhibit further corrosion.
Reference electrode
Cathodic protection uses a reference electrode like a saturated calomel electrode (SCE) or silver/silver chloride (Ag/AgCl) to measure and control the protected metal's potential, whereas anodic protection relies on maintaining the metal potential within a passive region using a reference electrode to prevent active corrosion.
Corrosion inhibitor
Cathodic protection uses sacrificial anodes or impressed current to prevent corrosion by making the metal surface a cathode, while anodic protection forms a passive oxide film on the metal surface, effectively acting as a corrosion inhibitor in highly acidic environments.
Mixed potential theory
Cathodic protection reduces corrosion by shifting the metal potential to a more negative value below the mixed potential, while anodic protection stabilizes a passive film by raising the potential above the active dissolution range based on mixed potential theory.
Cathodic protection vs Anodic protection Infographic
 njnir.com
njnir.com