Traceability in industrial engineering ensures comprehensive tracking of products throughout the supply chain, capturing detailed data on origin, processing, and distribution. Serialization assigns unique identifiers to individual items, enabling precise identification and authentication at any stage. While serialization enhances traceability by providing granular control, traceability encompasses a broader scope of tracking and data management for quality assurance and regulatory compliance.
Table of Comparison
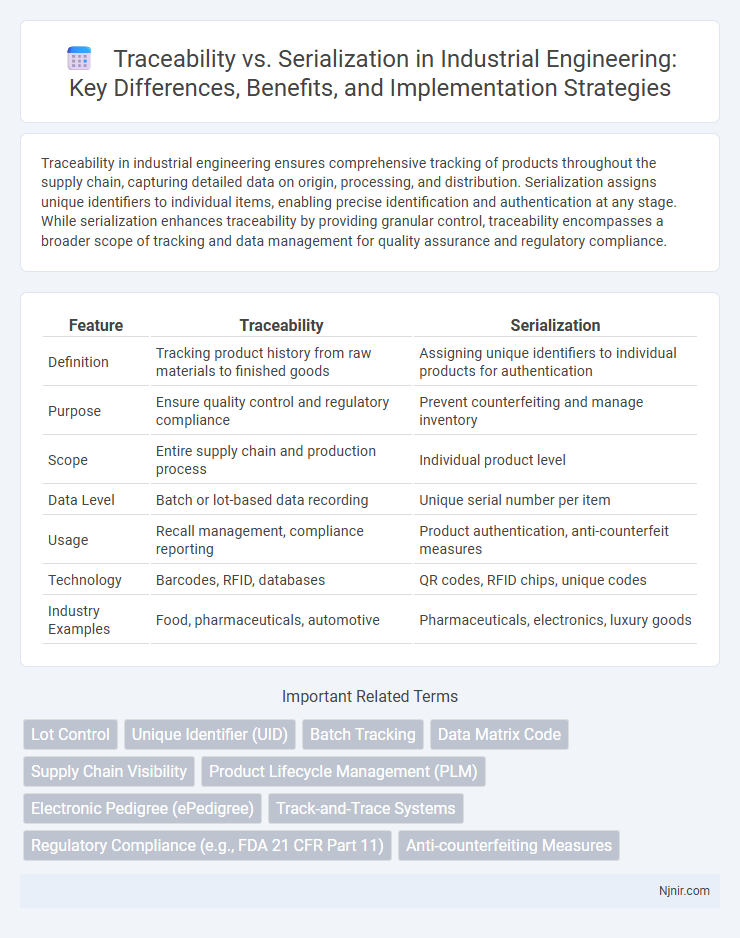
| Feature | Traceability | Serialization |
|---|---|---|
| Definition | Tracking product history from raw materials to finished goods | Assigning unique identifiers to individual products for authentication |
| Purpose | Ensure quality control and regulatory compliance | Prevent counterfeiting and manage inventory |
| Scope | Entire supply chain and production process | Individual product level |
| Data Level | Batch or lot-based data recording | Unique serial number per item |
| Usage | Recall management, compliance reporting | Product authentication, anti-counterfeit measures |
| Technology | Barcodes, RFID, databases | QR codes, RFID chips, unique codes |
| Industry Examples | Food, pharmaceuticals, automotive | Pharmaceuticals, electronics, luxury goods |
Introduction to Traceability and Serialization
Traceability involves tracking the history, application, or location of a product through recorded data, ensuring transparency and accountability across supply chains. Serialization assigns unique identifiers to individual products or batches, enabling precise tracking and authentication. Both concepts are crucial in industries like pharmaceuticals and food to enhance safety, prevent counterfeiting, and comply with regulatory standards.
Key Definitions: Traceability vs Serialization
Traceability refers to the ability to track the history, application, or location of a product through recorded data throughout the supply chain, ensuring transparency and accountability. Serialization involves assigning a unique identifier, often a serial number or barcode, to individual products or batches for precise tracking and authentication. While serialization is a method used to achieve traceability, traceability encompasses a broader scope of monitoring product movement and status beyond just unique identification.
The Role of Traceability in Industrial Engineering
Traceability in industrial engineering ensures the precise tracking of components and processes throughout the production lifecycle, enabling quality control and compliance with industry standards. It facilitates the identification of defects and the implementation of corrective actions by maintaining detailed records of each manufacturing step. Unlike serialization, which assigns unique identifiers to individual products, traceability integrates these identifiers into a comprehensive system that monitors the entire supply chain and production history.
Serialization: Concepts and Industrial Applications
Serialization is the process of assigning unique identifiers to individual products or components, enabling precise tracking and authenticity verification throughout the supply chain. Industrial applications of serialization include pharmaceuticals, electronics, and food industries, where it enhances anti-counterfeiting measures, regulatory compliance, and inventory management. Implementing serialization systems involves technologies such as barcodes, RFID, and QR codes to ensure product-level visibility and traceability.
Comparative Analysis: Traceability and Serialization
Traceability captures the complete history, location, and use of products through the supply chain, enabling comprehensive data tracking from raw materials to end consumers. Serialization involves assigning unique identifiers or codes to individual items or batches for precise identification and authentication. While traceability provides a broad overview of product movement and lifecycle, serialization focuses on item-level verification, improving anti-counterfeiting measures and regulatory compliance.
Benefits of Implementing Traceability Systems
Implementing traceability systems provides enhanced product transparency that enables companies to track components from origin to end-user, reducing risks of counterfeit goods and improving recall efficiency. These systems increase supply chain accountability by capturing detailed transaction data, which aids regulatory compliance and boosts consumer trust. Traceability also facilitates faster problem resolution and inventory management, leading to reduced operational costs and improved product quality control.
Advantages of Serialization in Manufacturing
Serialization in manufacturing enhances product traceability by assigning unique identifiers to each item, enabling precise tracking throughout the supply chain. This granular-level data helps prevent counterfeiting, ensures regulatory compliance, and facilitates efficient recalls by quickly isolating affected batches. Improved inventory management and increased customer trust are direct benefits, driving overall operational efficiency and brand protection.
Challenges in Adopting Traceability and Serialization
Challenges in adopting traceability and serialization include high implementation costs, complex integration with existing systems, and data management difficulties. Lack of standardized protocols across industries hinders seamless interoperability and increases compliance risks with regulatory requirements like the FDA and EU Falsified Medicines Directive. Ensuring data accuracy and security remains critical to maintain product integrity and prevent counterfeiting throughout supply chain operations.
Regulatory Requirements and Compliance Standards
Traceability and serialization are critical components in meeting regulatory requirements and compliance standards within industries such as pharmaceuticals, food, and electronics. Serialization involves assigning unique identifiers to individual products, enabling precise tracking and verification across the supply chain, while traceability ensures the ability to track the history, location, and application of these products throughout their lifecycle. Compliance with standards such as the Drug Supply Chain Security Act (DSCSA), FDA regulations, and ISO 9001 relies heavily on effective implementation of both serialization and traceability to prevent counterfeiting, enhance product recalls, and guarantee consumer safety.
Future Trends in Traceability and Serialization Technologies
Future trends in traceability and serialization technologies emphasize blockchain integration for enhanced data security and transparency across supply chains. Advanced IoT sensors and AI-driven analytics are increasingly deployed to provide real-time product tracking and predictive insights. Standardization efforts, such as GS1 digital link adoption, promote interoperability and global scalability in traceability systems.
Lot Control
Lot Control enhances traceability by grouping products under a single identifier, enabling efficient tracking and management throughout the supply chain without requiring individual serialization.
Unique Identifier (UID)
Serialization assigns a Unique Identifier (UID) to individual products for precise tracking, while traceability uses UIDs to monitor product history and movement across the supply chain.
Batch Tracking
Batch tracking enhances traceability by monitoring and recording production data for specific batches, enabling efficient identification, recall, and quality control throughout the supply chain.
Data Matrix Code
Data Matrix Code enhances traceability by encoding unique serialized data on products for efficient tracking, verification, and anti-counterfeiting in supply chains.
Supply Chain Visibility
Serialization enhances supply chain visibility by assigning unique identifiers to individual products, enabling precise traceability of their origin, movement, and status throughout the supply chain.
Product Lifecycle Management (PLM)
Traceability in Product Lifecycle Management (PLM) ensures comprehensive tracking of a product's history and changes, while serialization provides unique identification for individual items to enhance supply chain transparency and anti-counterfeiting measures.
Electronic Pedigree (ePedigree)
Electronic Pedigree (ePedigree) ensures traceability by documenting the complete lifecycle and ownership history of pharmaceutical products, while serialization assigns unique identifiers to each unit for precise tracking and authentication.
Track-and-Trace Systems
Track-and-trace systems enhance supply chain transparency by using serialization to assign unique identifiers to individual products and traceability to record their movement and history across all checkpoints.
Regulatory Compliance (e.g., FDA 21 CFR Part 11)
Traceability ensures accurate tracking of product history and movement, while serialization uniquely identifies each unit, both critical for meeting FDA 21 CFR Part 11 regulatory compliance through secure, auditable electronic records.
Anti-counterfeiting Measures
Serialization provides unique product identifiers enabling precise traceability, which significantly enhances anti-counterfeiting measures by allowing authentication at every supply chain stage.
Traceability vs Serialization Infographic
 njnir.com
njnir.com