Two-dimensional (2D) materials exhibit unique properties such as high surface area, exceptional mechanical strength, and superior electronic conductivity compared to their three-dimensional (3D) counterparts. The atomic thickness of 2D materials allows for enhanced flexibility and tunability, making them ideal for applications in nanoelectronics, sensors, and energy storage devices. In contrast, 3D materials offer bulk stability and well-established manufacturing processes but lack the distinct quantum confinement effects and surface phenomena inherent to 2D structures.
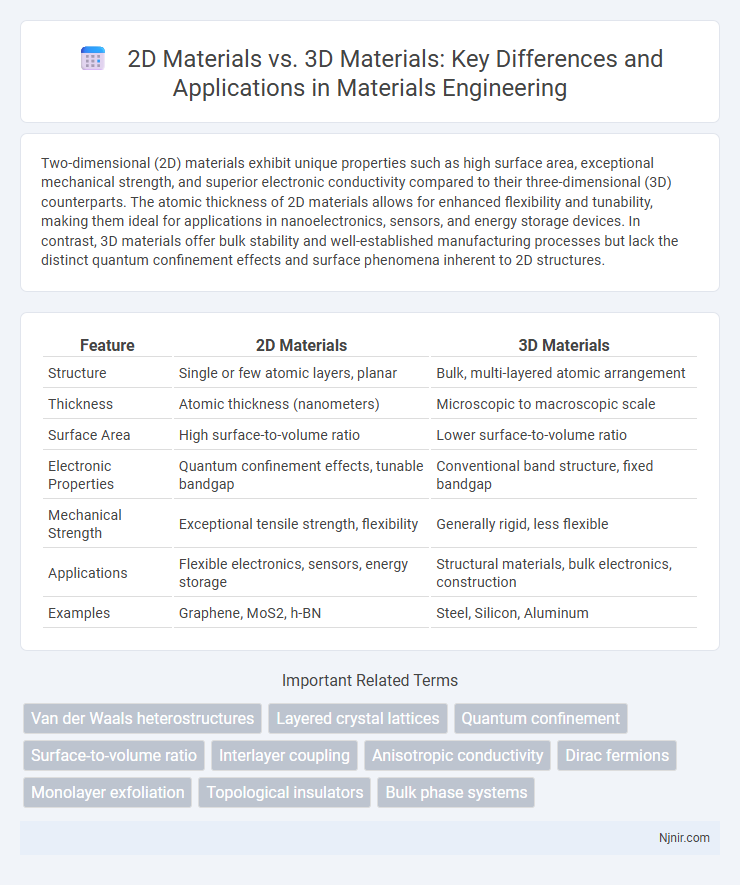
Table of Comparison
| Feature | 2D Materials | 3D Materials |
|---|---|---|
| Structure | Single or few atomic layers, planar | Bulk, multi-layered atomic arrangement |
| Thickness | Atomic thickness (nanometers) | Microscopic to macroscopic scale |
| Surface Area | High surface-to-volume ratio | Lower surface-to-volume ratio |
| Electronic Properties | Quantum confinement effects, tunable bandgap | Conventional band structure, fixed bandgap |
| Mechanical Strength | Exceptional tensile strength, flexibility | Generally rigid, less flexible |
| Applications | Flexible electronics, sensors, energy storage | Structural materials, bulk electronics, construction |
| Examples | Graphene, MoS2, h-BN | Steel, Silicon, Aluminum |
Introduction to 2D and 3D Materials
2D materials consist of a single layer of atoms arranged in a two-dimensional plane, offering unique electronic, mechanical, and thermal properties distinct from their 3D counterparts, which have atoms bonded in three-dimensional lattices. Graphene, molybdenum disulfide (MoS2), and hexagonal boron nitride are prominent examples of 2D materials, exhibiting exceptional conductivity and flexibility. In contrast, 3D materials like silicon and metals possess bulk properties governed by their extended atomic networks, resulting in different applications in electronics, structural components, and thermal management.
Structural Differences: Atomic Arrangements
2D materials consist of a single or few atomic layers arranged in a planar lattice, exhibiting strong in-plane covalent bonding and weak van der Waals forces between layers, leading to unique mechanical and electronic properties. In contrast, 3D materials feature atoms arranged in a bulk lattice with strong covalent or metallic bonds extending in all three spatial dimensions, resulting in isotropic properties. These fundamental differences in atomic arrangements influence the materials' band structures, conductivity, and optical behavior, crucial for applications in nanoelectronics and photonics.
Electronic Properties Comparison
2D materials, such as graphene and transition metal dichalcogenides (TMDCs), exhibit unique electronic properties including high electron mobility, tunable bandgaps, and strong spin-orbit coupling compared to their 3D counterparts. Unlike 3D materials, which typically have bulk electronic states and fixed band structures, 2D materials allow for enhanced quantum confinement effects and reduced dielectric screening, leading to stronger excitonic effects and improved charge carrier dynamics. These distinctions make 2D materials ideal for next-generation electronic devices, flexible electronics, and nanoscale transistors with superior performance metrics.
Mechanical Strength and Flexibility
2D materials such as graphene exhibit exceptional mechanical strength due to their atomic-scale thickness and highly ordered lattice structures, outperforming many traditional 3D materials in tensile strength. The inherent flexibility of 2D materials allows them to bend and stretch without fracturing, making them ideal for applications in flexible electronics and wearable devices. In contrast, 3D materials generally provide greater bulk strength but lack the remarkable flexibility and strength-to-weight ratio found in 2D counterparts.
Thermal Conductivity and Stability
2D materials such as graphene exhibit exceptionally high thermal conductivity, often exceeding 3000 W/mK, due to strong in-plane covalent bonding and reduced phonon scattering, whereas 3D materials like silicon have lower thermal conductivity, generally around 150 W/mK. The atomic thinness of 2D materials contributes to unique thermal management properties but also presents challenges in chemical and mechanical stability compared to bulk 3D materials which offer greater structural robustness. Stability in 2D materials can be enhanced through substrate support or functionalization, whereas 3D materials maintain inherent stability due to their volumetric nature and isotropic bonding networks.
Synthesis Methods for 2D and 3D Materials
Synthesis methods for 2D materials often involve techniques such as chemical vapor deposition (CVD), mechanical exfoliation, and liquid-phase exfoliation, enabling the production of atomically thin layers with unique electronic and mechanical properties. In contrast, 3D materials are typically synthesized using bulk methods like solid-state reactions, melting, and hydrothermal synthesis, which focus on forming larger, volumetric crystalline structures. The precision and scalability of 2D material synthesis directly influence their potential applications in electronics, optoelectronics, and energy storage, differentiating them from conventional 3D counterparts.
Surface Area and Reactivity Variations
2D materials such as graphene and transition metal dichalcogenides exhibit significantly higher surface area-to-volume ratios compared to traditional 3D materials, enhancing their surface reactivity and catalytic potential. The atomic-scale thickness of 2D materials exposes every atom to the surface environment, leading to increased chemical activity and sensitivity. In contrast, 3D materials have bulk structures where surface atoms constitute a smaller fraction, resulting in lower overall reactivity and limited active sites for surface interactions.
Key Applications in Industry
2D materials, such as graphene and molybdenum disulfide, exhibit exceptional electrical conductivity and mechanical flexibility, making them ideal for advanced electronics, flexible displays, and energy storage solutions in the semiconductor and renewable energy industries. In contrast, 3D materials offer robustness and structural integrity essential for construction, automotive manufacturing, and aerospace engineering, where strength, durability, and thermal stability are critical. Industries leverage 2D materials for miniaturized, high-performance components, while relying on 3D materials for large-scale, load-bearing applications.
Challenges and Limitations
Two-dimensional (2D) materials face challenges such as scalability issues in large-area synthesis, structural defects, and difficulties in maintaining stability under ambient conditions, limiting their practical application compared to bulk three-dimensional (3D) materials. Conversely, 3D materials benefit from well-established fabrication techniques but encounter limitations related to bulkiness, lower surface-to-volume ratios, and reduced electronic tunability. Both 2D and 3D materials require advancements in integration methods and defect control to overcome performance bottlenecks in electronics, photonics, and energy storage applications.
Future Trends in Materials Engineering
2D materials such as graphene and transition metal dichalcogenides offer exceptional mechanical strength, electrical conductivity, and flexibility compared to traditional 3D materials, driving innovation in electronics, sensors, and energy storage. Future trends in materials engineering emphasize the integration of 2D materials into hybrid composites and nanoelectronics to achieve enhanced performance at reduced weight and size. Advances in scalable synthesis methods and atomic-level control are crucial for industrial adoption and the development of next-generation devices with unprecedented functionalities.
Van der Waals heterostructures
Van der Waals heterostructures formed by stacking 2D materials exhibit unique electronic, optical, and mechanical properties distinct from bulk 3D materials due to their atomically thin layers and weak interlayer interactions.
Layered crystal lattices
Layered crystal lattices in 2D materials enable unique electronic and mechanical properties absent in bulk 3D materials due to their atomic-scale thickness and strong in-plane bonding combined with weak out-of-plane van der Waals interactions.
Quantum confinement
Quantum confinement in 2D materials drastically alters their electronic and optical properties compared to 3D materials by restricting electron movement to two dimensions, resulting in enhanced tunable bandgaps and stronger excitonic effects.
Surface-to-volume ratio
2D materials exhibit a significantly higher surface-to-volume ratio compared to 3D materials, enhancing their chemical reactivity and surface-dependent properties.
Interlayer coupling
2D materials exhibit significantly weaker interlayer coupling compared to 3D materials, leading to unique electronic, optical, and mechanical properties crucial for applications in nanoelectronics and optoelectronics.
Anisotropic conductivity
2D materials exhibit pronounced anisotropic conductivity due to their layer-dependent electronic structures, contrasting with the typically isotropic conductivity of bulk 3D materials.
Dirac fermions
Dirac fermions in 2D materials like graphene exhibit unique massless charge carrier behavior and high electron mobility, contrasting with the conventional massive fermions found in 3D materials.
Monolayer exfoliation
Monolayer exfoliation enables the isolation of atomically thin 2D materials from bulk 3D counterparts, enhancing electronic, optical, and mechanical properties for advanced nanotechnology applications.
Topological insulators
Topological insulators exhibit distinct electronic properties in 2D materials due to quantum confinement effects, whereas 3D materials present bulk insulating behavior with conducting surface states enabling unique spintronic applications.
Bulk phase systems
Bulk phase systems in 3D materials exhibit volumetric atomic arrangements enabling inherent mechanical strength and thermal conductivity, whereas 2D materials possess planar atomic layers offering unique electronic, optical, and surface properties despite limited bulk atomic interactions.
2D Materials vs 3D Materials Infographic
 njnir.com
njnir.com