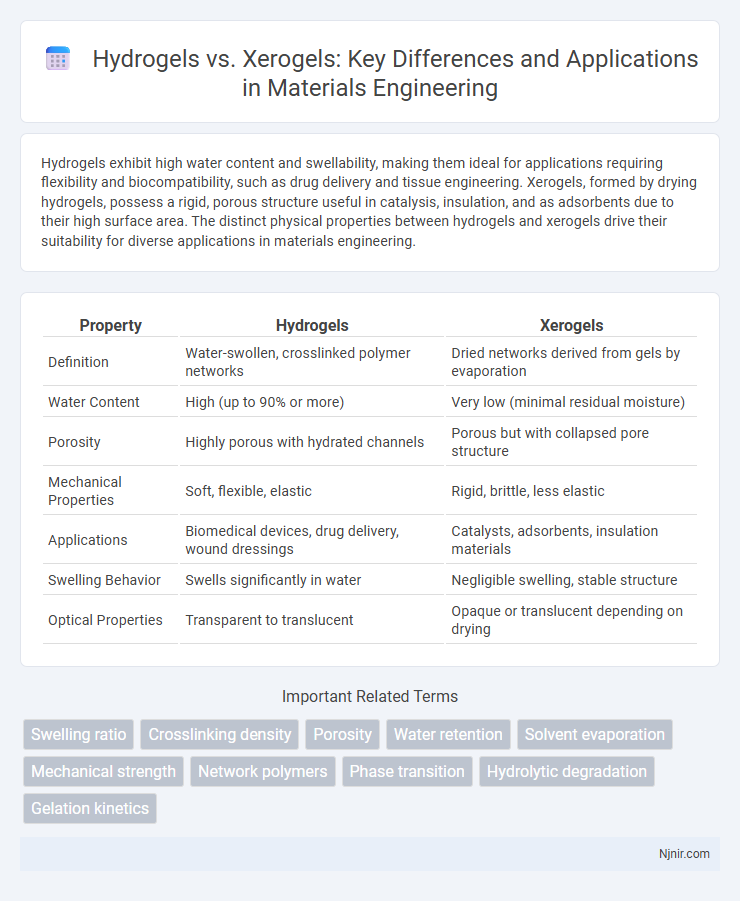
Hydrogels exhibit high water content and swellability, making them ideal for applications requiring flexibility and biocompatibility, such as drug delivery and tissue engineering. Xerogels, formed by drying hydrogels, possess a rigid, porous structure useful in catalysis, insulation, and as adsorbents due to their high surface area. The distinct physical properties between hydrogels and xerogels drive their suitability for diverse applications in materials engineering.
Table of Comparison
| Property | Hydrogels | Xerogels |
|---|---|---|
| Definition | Water-swollen, crosslinked polymer networks | Dried networks derived from gels by evaporation |
| Water Content | High (up to 90% or more) | Very low (minimal residual moisture) |
| Porosity | Highly porous with hydrated channels | Porous but with collapsed pore structure |
| Mechanical Properties | Soft, flexible, elastic | Rigid, brittle, less elastic |
| Applications | Biomedical devices, drug delivery, wound dressings | Catalysts, adsorbents, insulation materials |
| Swelling Behavior | Swells significantly in water | Negligible swelling, stable structure |
| Optical Properties | Transparent to translucent | Opaque or translucent depending on drying |
Introduction to Hydrogels and Xerogels
Hydrogels are three-dimensional polymer networks capable of retaining large amounts of water, making them highly effective for biomedical applications such as drug delivery and tissue engineering. Xerogels, on the other hand, are derived from hydrogels by removing water through drying, resulting in a porous and solid structure often used in adsorption and catalysis. The fundamental difference lies in their water content and physical properties, influencing their practical uses across various industries.
Fundamental Differences Between Hydrogels and Xerogels
Hydrogels are three-dimensional polymer networks capable of retaining large amounts of water, rendering them highly swellable and soft, while xerogels are the dried form of gels with minimal to no water content, resulting in a porous and rigid structure. The fundamental difference lies in their water content and physical state: hydrogels maintain hydration and flexibility, whereas xerogels exhibit structural shrinkage and increased brittleness due to dehydration. These distinctions impact their applications, with hydrogels favored in biomedical fields requiring moisture retention and xerogels used in areas needing porous, stable materials like catalysts or adsorbents.
Synthesis and Fabrication Methods
Hydrogels are synthesized through polymerization of hydrophilic monomers using techniques such as free-radical polymerization, crosslinking agents, or radiation methods, enabling the formation of three-dimensional networks capable of absorbing significant water. Xerogels, derived by drying hydrogels through processes like ambient drying or supercritical drying, retain the porous structure but lose most of their water content, resulting in a dense, brittle solid. Fabrication methods for hydrogels often emphasize maintaining biocompatibility and elasticity, while xerogel synthesis focuses on preserving pore volume and surface area critical for applications in catalysis and adsorption.
Structural and Morphological Characteristics
Hydrogels exhibit a three-dimensional network of hydrophilic polymer chains that imbibe large amounts of water, resulting in a soft, flexible, and highly porous structure. Xerogels, formed by drying hydrogels, possess a dense, rigid morphology with significantly reduced pore volume and surface area due to the collapse of the polymer network. The structural transition from hydrated hydrogel to dehydrated xerogel alters mechanical properties and porosity, impacting applications in drug delivery and tissue engineering.
Mechanical and Physical Properties Comparison
Hydrogels exhibit high water content with a 3D polymer network, resulting in excellent elasticity, flexibility, and softness, whereas xerogels are dried forms with low moisture, leading to rigid, brittle structures and increased hardness. The mechanical strength of hydrogels is generally lower due to their swollen state, while xerogels demonstrate higher compressive strength and reduced swelling capacity. Physically, hydrogels maintain a porous, hydrated matrix ideal for biomedical applications, whereas xerogels possess a denser, less permeable structure suitable for insulation and catalyst supports.
Water Absorption and Swelling Behavior
Hydrogels exhibit significant water absorption and swelling behavior due to their three-dimensional polymer networks capable of retaining large amounts of water, often swelling to several times their dry weight. Xerogels, in contrast, are dehydrated forms of gels with minimal water content, resulting in limited swelling and lower water absorption. The porous structure of hydrogels facilitates rapid and extensive fluid uptake, while xerogels typically require rehydration to regain any swelling capacity.
Applications in Biomedical Engineering
Hydrogels, known for their high water content and biocompatibility, are extensively used in wound dressings, drug delivery systems, and tissue engineering scaffolds, where their capacity to mimic natural extracellular matrices promotes cell growth and healing. Xerogels, derived from dried hydrogels, offer high porosity and mechanical stability ideal for implant coatings, biosensors, and controlled release applications requiring structural integrity under physiological conditions. Advances in biomedical engineering exploit hydrogels for their swelling properties and xerogels for their durability, optimizing both materials to enhance patient-specific therapeutic outcomes.
Environmental and Industrial Applications
Hydrogels exhibit exceptional water-retention capabilities, making them ideal for environmental applications such as soil moisture management and controlled-release fertilizers, enhancing agricultural efficiency and reducing water waste. Xerogels, with their porous and lightweight structure, are widely utilized in industrial processes including catalysis, insulation, and adsorption of pollutants, offering superior surface area for contaminant capture and thermal regulation. The distinct physicochemical properties of hydrogels and xerogels enable targeted application in sectors focused on sustainability and resource optimization.
Limitations and Challenges
Hydrogels often face limitations such as poor mechanical strength, limited thermal stability, and susceptibility to drying out, which restrict their use in long-term biomedical applications. Xerogels, while exhibiting enhanced mechanical properties and better thermal resistance, suffer from brittleness and reduced swelling capacity, constraining their functionality in drug delivery and tissue engineering. Both materials present challenges in achieving the ideal balance between flexibility, durability, and biocompatibility for specific industrial and medical applications.
Future Trends and Advancement in Hydrogel and Xerogel Research
Hydrogel and xerogel research is advancing rapidly with significant focus on nanoscale engineering and multifunctional applications such as drug delivery, wound healing, and environmental sensors. Emerging trends emphasize biodegradable and stimuli-responsive hydrogels integrated with smart materials to enhance biocompatibility and targeted therapy, while xerogels are being developed for improved thermal insulation, catalysis, and energy storage through controlled porosity and structural tuning. Innovations in hybrid hydrogel-xerogel composites and 3D printing techniques promise to revolutionize personalized medicine and sustainable materials, driving the next generation of biomaterials and high-performance industrial applications.
Swelling ratio
Hydrogels exhibit a significantly higher swelling ratio compared to xerogels due to their porous polymer network that absorbs and retains large amounts of water.
Crosslinking density
Hydrogels exhibit high crosslinking density resulting in greater water retention and elasticity, whereas xerogels have lower crosslinking density, leading to rigid, porous structures with reduced moisture content.
Porosity
Hydrogels exhibit high porosity with interconnected water-filled networks, while xerogels possess lower porosity due to pore collapse during drying, resulting in a denser, less permeable structure.
Water retention
Hydrogels retain up to 90% water by weight due to their cross-linked polymer networks, whereas xerogels have minimal water retention because they are dried gels with porous, rigid structures.
Solvent evaporation
Hydrogels retain high solvent content with minimal evaporation due to their three-dimensional polymer networks, whereas xerogels undergo extensive solvent evaporation leading to porous, solid structures with reduced moisture.
Mechanical strength
Hydrogels exhibit lower mechanical strength due to high water content and network flexibility, whereas xerogels demonstrate enhanced mechanical strength resulting from water removal and densified porous structure.
Network polymers
Hydrogels are three-dimensional polymer networks that retain water within their structure, while xerogels are dehydrated forms of these networks exhibiting collapsed polymer matrices with reduced porosity.
Phase transition
Hydrogels undergo a reversible phase transition by absorbing water and swelling, whereas xerogels exhibit irreversible shrinkage and densification upon drying.
Hydrolytic degradation
Hydrogels exhibit faster hydrolytic degradation due to their high water content and swelling capacity, while xerogels degrade more slowly because of their dense, dehydrated network structure.
Gelation kinetics
Hydrogels exhibit faster gelation kinetics due to their higher water content enabling rapid polymer crosslinking, while xerogels show slower gelation rates constrained by reduced moisture and denser polymer networks.
Hydrogels vs Xerogels Infographic
 njnir.com
njnir.com