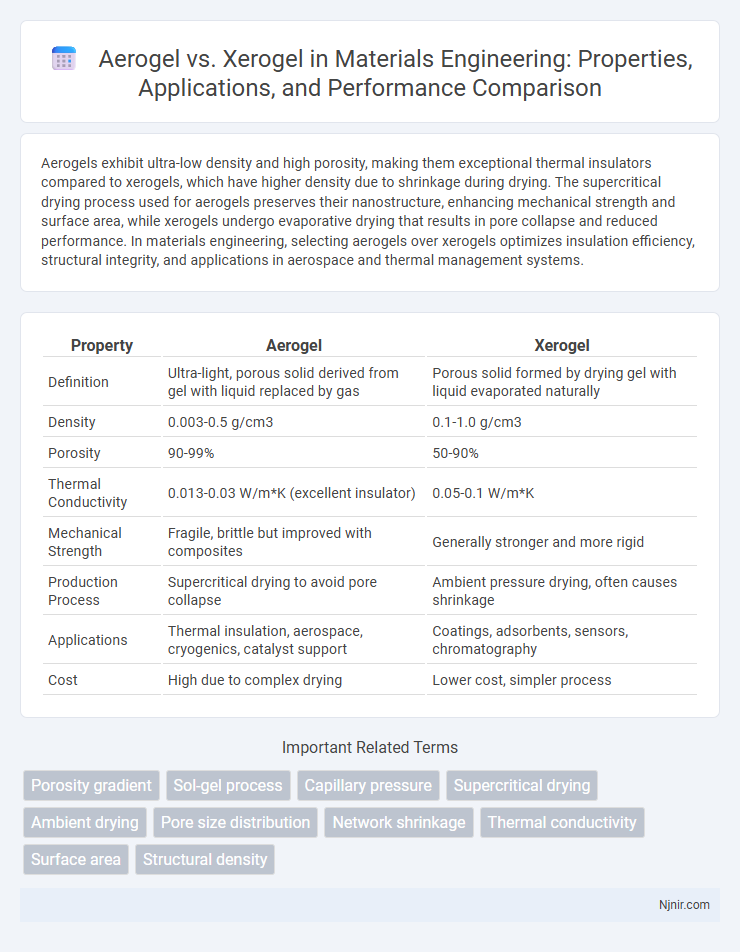
Aerogels exhibit ultra-low density and high porosity, making them exceptional thermal insulators compared to xerogels, which have higher density due to shrinkage during drying. The supercritical drying process used for aerogels preserves their nanostructure, enhancing mechanical strength and surface area, while xerogels undergo evaporative drying that results in pore collapse and reduced performance. In materials engineering, selecting aerogels over xerogels optimizes insulation efficiency, structural integrity, and applications in aerospace and thermal management systems.
Table of Comparison
| Property | Aerogel | Xerogel |
|---|---|---|
| Definition | Ultra-light, porous solid derived from gel with liquid replaced by gas | Porous solid formed by drying gel with liquid evaporated naturally |
| Density | 0.003-0.5 g/cm3 | 0.1-1.0 g/cm3 |
| Porosity | 90-99% | 50-90% |
| Thermal Conductivity | 0.013-0.03 W/m*K (excellent insulator) | 0.05-0.1 W/m*K |
| Mechanical Strength | Fragile, brittle but improved with composites | Generally stronger and more rigid |
| Production Process | Supercritical drying to avoid pore collapse | Ambient pressure drying, often causes shrinkage |
| Applications | Thermal insulation, aerospace, cryogenics, catalyst support | Coatings, adsorbents, sensors, chromatography |
| Cost | High due to complex drying | Lower cost, simpler process |
Introduction to Aerogel and Xerogel
Aerogel and xerogel are both highly porous materials derived from gels through different drying processes, with aerogel formed via supercritical drying and xerogel through ambient pressure drying. Aerogels possess extremely low density, high surface area, and excellent thermal insulation properties due to their nanostructured, open-cell framework. Xerogels tend to have higher density and reduced porosity compared to aerogels, impacting their mechanical strength and permeability characteristics.
Definition and Formation Processes
Aerogel is an ultralight porous material derived from a gel in which the liquid component is replaced with gas through supercritical drying, preserving its three-dimensional network. Xerogel forms when a gel undergoes evaporative drying, causing significant shrinkage and pore collapse due to surface tension effects. The key difference lies in their formation: aerogels maintain high porosity and low density by avoiding liquid-gas phase transition stress, whereas xerogels exhibit denser, less porous structures resulting from ambient pressure drying.
Structural Differences
Aerogels exhibit a highly porous, open-cell structure with pore sizes typically ranging from 2 to 50 nanometers, resulting from supercritical drying that preserves the gel network without collapsing. Xerogels, by contrast, undergo ambient pressure drying, causing significant shrinkage and densification, which leads to smaller pores and a more compact, less porous microstructure. These structural disparities critically influence their thermal insulation, mechanical strength, and surface area characteristics.
Physical and Chemical Properties
Aerogels exhibit ultra-low density, high porosity (up to 99.8%), and excellent thermal insulation due to their nanoporous structure, while xerogels possess a denser structure resulting from solvent evaporation causing shrinkage. Chemically, aerogels maintain high surface area and minimal chemical alteration during supercritical drying, preserving silanol groups, whereas xerogels often experience partial condensation and reduced surface area due to capillary stress. The distinct drying processes yield aerogels with superior mechanical flexibility and optical transparency compared to the brittle, less porous xerogels.
Thermal Insulation Performance
Aerogels exhibit superior thermal insulation performance compared to xerogels due to their highly porous structure with nanoscale pores, resulting in extremely low thermal conductivity values typically around 0.013 W/m*K. Xerogels, formed through ambient pressure drying, have higher density and larger pore sizes, which increase heat transfer and reduce insulation efficiency. Aerogels are preferred in applications requiring advanced thermal barriers, such as aerospace and building insulation, because they provide better resistance to heat flow and maintain structural integrity under thermal stress.
Mechanical Strength Comparison
Aerogels exhibit significantly higher mechanical strength compared to xerogels due to their highly porous yet interconnected nanostructure, which provides enhanced load distribution and resilience. Xerogels tend to be more brittle and fragile because the drying process causes collapse of the pore structure, reducing their ability to withstand mechanical stress. Advanced testing reveals that aerogels can endure compressive stresses up to 10 MPa, whereas xerogels typically fail at much lower stress levels, often below 1 MPa.
Applications in Industry
Aerogel's ultra-low density and exceptional thermal insulation make it ideal for aerospace, automotive, and building industries where lightweight and heat resistance are critical. Xerogel finds extensive use in adsorption and catalysis processes due to its porous structure and stability, particularly in environmental remediation and chemical sensors. Both materials enable advancements in electronics, energy storage, and filtration, but aerogel's superior insulation often drives preference in thermal management applications.
Environmental Impact and Sustainability
Aerogels exhibit superior environmental sustainability due to their ultra-low density and high thermal insulation properties, significantly reducing energy consumption in buildings and industrial applications. Xerogels, while similar in composition, have a higher density that limits their insulation efficiency and increases material usage, impacting resource sustainability. The production of aerogels often involves eco-friendly precursors and low-impact synthesis methods, making them a more sustainable choice compared to xerogels in environmentally conscious applications.
Cost and Scalability Considerations
Aerogels typically have higher production costs due to their energy-intensive supercritical drying processes, limiting large-scale manufacturing compared to xerogels, which use ambient pressure drying and are more cost-effective for mass production. Xerogels offer better scalability with simpler, less expensive equipment, making them viable for industrial applications requiring bulk quantities. Despite lower cost, xerogels may exhibit compromises in material properties, while aerogels justify their premium pricing through superior insulating performance and lower density.
Future Developments and Emerging Trends
Aerogel and xerogel technologies are advancing rapidly with a focus on enhancing thermal insulation and lightweight structural applications in aerospace and construction industries. Emerging trends include the development of hybrid aerogel-xerogel composites that improve mechanical strength and reduce production costs. Future developments emphasize scalable manufacturing techniques and environmentally friendly synthesis methods to broaden commercial adoption.
Porosity gradient
Aerogels exhibit a more uniform porosity gradient with ultra-high pore volume and low density, whereas xerogels have a less controlled porosity gradient due to shrinkage during drying, resulting in denser structures.
Sol-gel process
Aerogels and xerogels are both porous materials derived from the sol-gel process, with aerogels retaining their nanostructure through supercritical drying to achieve high porosity and low density, whereas xerogels undergo ambient drying resulting in shrinkage and higher density.
Capillary pressure
Aerogels exhibit significantly lower capillary pressure compared to xerogels due to their highly porous, low-density structure formed through supercritical drying, which prevents pore collapse and reduces surface tension effects.
Supercritical drying
Supercritical drying preserves the porous network of aerogels by removing liquid without surface tension effects, whereas xerogels experience pore collapse due to evaporation-induced capillary forces during drying.
Ambient drying
Aerogel offers superior insulation with minimal shrinkage and porosity retention compared to xerogel, which typically undergoes ambient drying resulting in higher density and reduced performance.
Pore size distribution
Aerogels typically exhibit a narrower and more uniform pore size distribution ranging from 2 to 50 nanometers, while xerogels have a broader and less controlled pore size distribution often extending beyond 100 nanometers due to shrinkage during drying.
Network shrinkage
Aerogels exhibit minimal network shrinkage due to supercritical drying, whereas xerogels undergo significant shrinkage caused by capillary forces during evaporative drying.
Thermal conductivity
Aerogel exhibits significantly lower thermal conductivity, typically around 0.01 W/m*K, compared to xerogel's higher values near 0.03 W/m*K, making aerogel superior for thermal insulation applications.
Surface area
Aerogels typically exhibit a higher surface area ranging from 600 to 1200 m2/g, whereas xerogels have a comparatively lower surface area of about 200 to 600 m2/g due to pore collapse during drying.
Structural density
Aerogels exhibit significantly lower structural density compared to xerogels due to their highly porous, nanoscale network formed through supercritical drying processes that minimize pore collapse.
aerogel vs xerogel Infographic
 njnir.com
njnir.com