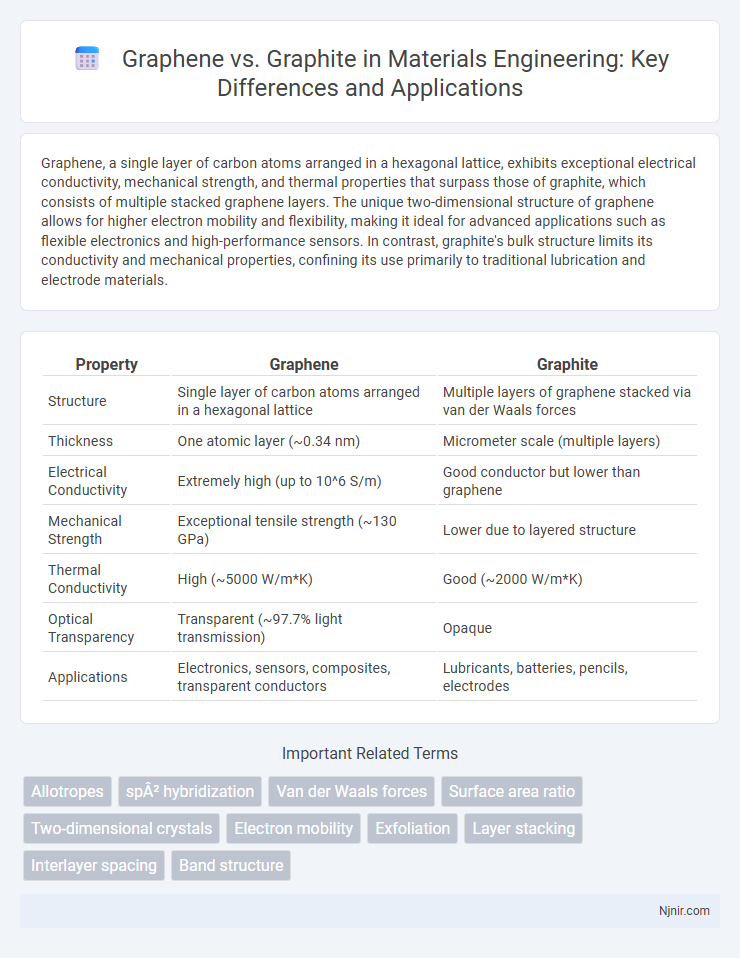
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, exhibits exceptional electrical conductivity, mechanical strength, and thermal properties that surpass those of graphite, which consists of multiple stacked graphene layers. The unique two-dimensional structure of graphene allows for higher electron mobility and flexibility, making it ideal for advanced applications such as flexible electronics and high-performance sensors. In contrast, graphite's bulk structure limits its conductivity and mechanical properties, confining its use primarily to traditional lubrication and electrode materials.
Table of Comparison
| Property | Graphene | Graphite |
|---|---|---|
| Structure | Single layer of carbon atoms arranged in a hexagonal lattice | Multiple layers of graphene stacked via van der Waals forces |
| Thickness | One atomic layer (~0.34 nm) | Micrometer scale (multiple layers) |
| Electrical Conductivity | Extremely high (up to 10^6 S/m) | Good conductor but lower than graphene |
| Mechanical Strength | Exceptional tensile strength (~130 GPa) | Lower due to layered structure |
| Thermal Conductivity | High (~5000 W/m*K) | Good (~2000 W/m*K) |
| Optical Transparency | Transparent (~97.7% light transmission) | Opaque |
| Applications | Electronics, sensors, composites, transparent conductors | Lubricants, batteries, pencils, electrodes |
Introduction to Graphene and Graphite
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, known for its exceptional electrical conductivity, mechanical strength, and transparency. Graphite consists of multiple layers of graphene stacked together, bonded by weak van der Waals forces that allow layers to slide over each other, making it an excellent lubricant and electrically conductive material. The unique atomic structure of graphene distinguishes it from graphite, enabling advanced applications in electronics, composites, and energy storage.
Structural Differences Between Graphene and Graphite
Graphene consists of a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, creating a structure with exceptional electrical conductivity and mechanical strength. Graphite, on the other hand, is composed of multiple stacked layers of graphene held together by weak van der Waals forces, enabling easy layer separation and giving graphite its lubricating properties. The structural distinction between the single atomic layer in graphene and the multi-layered crystalline form in graphite fundamentally influences their electrical, thermal, and mechanical characteristics.
Electronic Properties Comparison
Graphene exhibits exceptional electronic properties due to its two-dimensional structure, allowing electrons to behave as massless Dirac fermions with extremely high mobility exceeding 200,000 cm2/V*s at room temperature. In contrast, graphite, composed of stacked graphene layers, shows lower electron mobility around 10,000 cm2/V*s due to interlayer interactions and scattering effects. The zero bandgap in graphene results in ambipolar electric field effect, while graphite's semimetallic nature provides a small overlap between conduction and valence bands, influencing its bulk conductivity.
Mechanical Strength and Flexibility
Graphene exhibits exceptional mechanical strength with a tensile strength of approximately 130 GPa, surpassing graphite, whose layered structure results in weaker interlayer bonding and lower mechanical robustness. Graphene's single-atom thickness and planar hexagonal lattice enable outstanding flexibility without compromising its strength, while graphite's multiple layers limit its flexibility due to interlayer van der Waals forces. The superior mechanical properties of graphene make it ideal for applications requiring both high strength and flexibility, unlike bulk graphite.
Thermal Conductivity and Stability
Graphene exhibits extraordinary thermal conductivity of up to 5000 W/mK, surpassing graphite's typical range of 150-250 W/mK due to its single-atom thickness and sp2-bonded carbon structure that promotes efficient phonon transport. Graphene maintains high thermal stability up to 600degC in air, whereas graphite, being bulk layered carbon, resists oxidation at temperatures around 600-900degC but shows lower thermal conductivity. These properties make graphene ideal for advanced heat dissipation applications, while graphite remains valuable for high-temperature stability in industrial processes.
Synthesis and Production Methods
Graphene is primarily synthesized using methods such as chemical vapor deposition (CVD), mechanical exfoliation, and liquid-phase exfoliation, which allow for the production of single or few-layer graphene sheets with high purity and controlled thickness. Graphite, on the other hand, is naturally occurring and commonly processed through mining and purification techniques, with synthetic graphite produced via high-temperature treatment of carbon-rich precursors in graphitization furnaces. The distinct production methods reflect graphene's need for precise layer control and structural integrity, whereas graphite's bulk form suits large-scale extraction and industrial applications.
Applications in Modern Materials Engineering
Graphene's exceptional electrical conductivity and mechanical strength enable its use in flexible electronics, high-performance batteries, and advanced sensors, outperforming traditional graphite. Graphite, primarily utilized as a lubricant and in electrodes for batteries and fuel cells, lacks the nanoscale properties of graphene that revolutionize thermal management and composite materials. Modern materials engineering increasingly favors graphene for its ability to enhance energy storage, catalysis, and wearable technology applications, driving innovation beyond graphite's conventional uses.
Environmental Impact and Sustainability
Graphene, derived from graphite, offers enhanced sustainability due to its potential for energy-efficient applications and longer lifecycle in electronics, reducing electronic waste. Graphite mining, however, poses environmental risks such as habitat destruction and pollution, while graphene production methods are evolving to minimize toxic chemical use and carbon footprint. Transitioning to graphene-based technologies promotes eco-friendly innovation by reducing reliance on traditional materials with higher environmental degradation.
Challenges in Commercialization
Graphene faces significant challenges in commercialization due to high production costs and difficulties in achieving consistent quality and scalability compared to graphite, which is abundant and inexpensive. The complexity of synthesizing defect-free graphene layers limits its integration into large-scale industrial applications. Overcoming these hurdles requires advancements in cost-effective manufacturing techniques and standardized quality control processes to unlock graphene's full commercial potential.
Future Trends in Graphene and Graphite Research
Graphene research is rapidly advancing with a focus on scalable production methods, improving material quality, and integrating graphene into flexible electronics, energy storage, and advanced composites. Graphite research is concentrating on enhancing purification processes and developing new graphite-derived materials for battery anodes and lubricants. Future trends highlight the synergy between graphene's exceptional conductivity and graphite's abundance, driving innovations in sustainable energy technologies and high-performance materials.
Allotropes
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, is an allotrope of graphite, which consists of multiple stacked graphene layers bonded weakly by van der Waals forces.
sp² hybridization
Graphene exhibits a single layer of sp2 hybridized carbon atoms arranged in a two-dimensional honeycomb lattice, whereas graphite consists of multiple stacked layers of graphene held together by weak van der Waals forces.
Van der Waals forces
Graphene exhibits significantly weaker Van der Waals forces compared to graphite due to its single-layer atomic structure, which results in enhanced electrical conductivity and mechanical strength.
Surface area ratio
Graphene offers an exceptionally high surface area ratio of approximately 2630 m2/g compared to graphite's significantly lower surface area of about 10 m2/g, making graphene vastly superior for applications requiring large surface exposure.
Two-dimensional crystals
Graphene, a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, exhibits exceptional electrical conductivity and mechanical strength compared to its three-dimensional counterpart, graphite, which consists of stacked layers of graphene sheets held together by weak van der Waals forces.
Electron mobility
Graphene exhibits electron mobility exceeding 200,000 cm2/V*s, vastly surpassing graphite's electron mobility, which typically ranges around 1,500 cm2/V*s.
Exfoliation
Graphene is produced through exfoliation of graphite, where individual atomic layers are separated to obtain single-layer or few-layer graphene sheets with exceptional electrical, mechanical, and thermal properties.
Layer stacking
Graphene consists of a single layer of carbon atoms arranged in a hexagonal lattice, while graphite is composed of multiple graphene layers stacked together with weak van der Waals forces between them.
Interlayer spacing
Graphene exhibits a single atomic layer structure with an interlayer spacing of approximately 0.335 nanometers in graphite, where multiple graphene layers stack through van der Waals forces.
Band structure
Graphene exhibits a unique zero-bandgap Dirac cone electronic structure enabling exceptional electrical conductivity, whereas graphite's band structure results in a finite bandgap due to interlayer interactions that limit carrier mobility.
graphene vs graphite Infographic
 njnir.com
njnir.com