Hydrogen embrittlement occurs when hydrogen atoms diffuse into metals, causing a loss of ductility and premature failure under tensile stress. Stress corrosion cracking arises from the combined effects of tensile stress and a corrosive environment, leading to crack initiation and propagation along specific crystallographic planes. Differentiating these phenomena is critical for selecting appropriate materials and preventive measures in mechanical design to enhance durability and safety.
Table of Comparison
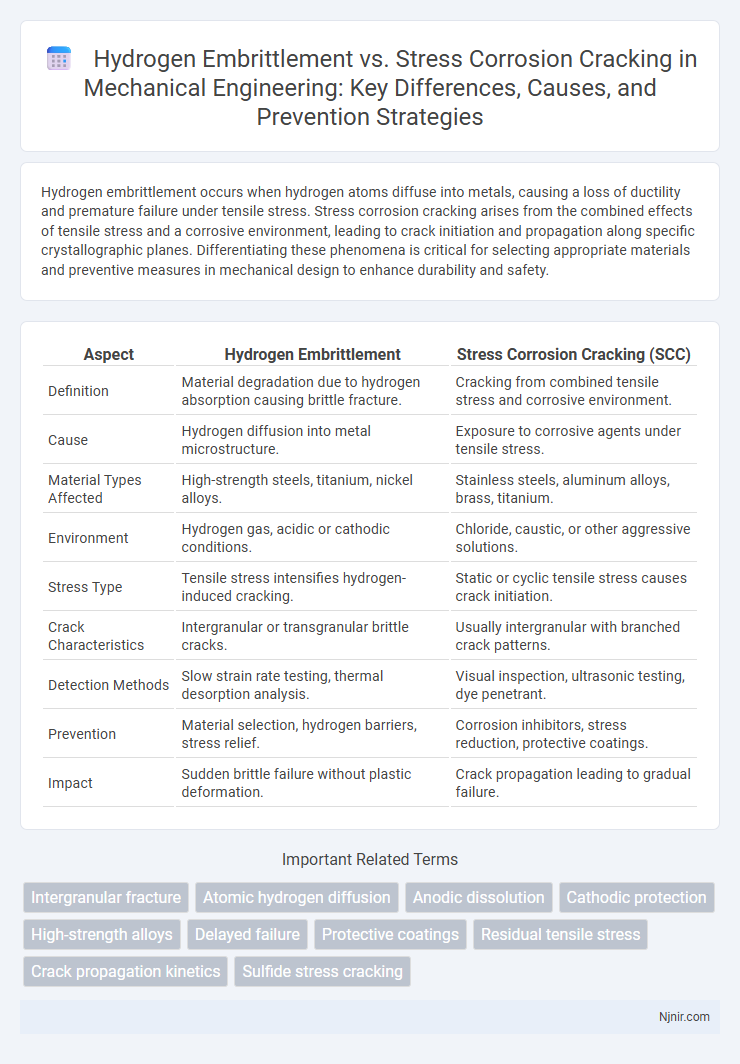
| Aspect | Hydrogen Embrittlement | Stress Corrosion Cracking (SCC) |
|---|---|---|
| Definition | Material degradation due to hydrogen absorption causing brittle fracture. | Cracking from combined tensile stress and corrosive environment. |
| Cause | Hydrogen diffusion into metal microstructure. | Exposure to corrosive agents under tensile stress. |
| Material Types Affected | High-strength steels, titanium, nickel alloys. | Stainless steels, aluminum alloys, brass, titanium. |
| Environment | Hydrogen gas, acidic or cathodic conditions. | Chloride, caustic, or other aggressive solutions. |
| Stress Type | Tensile stress intensifies hydrogen-induced cracking. | Static or cyclic tensile stress causes crack initiation. |
| Crack Characteristics | Intergranular or transgranular brittle cracks. | Usually intergranular with branched crack patterns. |
| Detection Methods | Slow strain rate testing, thermal desorption analysis. | Visual inspection, ultrasonic testing, dye penetrant. |
| Prevention | Material selection, hydrogen barriers, stress relief. | Corrosion inhibitors, stress reduction, protective coatings. |
| Impact | Sudden brittle failure without plastic deformation. | Crack propagation leading to gradual failure. |
Introduction to Hydrogen Embrittlement and Stress Corrosion Cracking
Hydrogen embrittlement (HE) occurs when metals, especially high-strength steels, absorb atomic hydrogen, leading to a significant loss of ductility and premature brittle failure under tensile stress. Stress corrosion cracking (SCC) involves the combined effects of tensile stress and a corrosive environment, causing crack initiation and propagation in susceptible alloys such as stainless steel and aluminum. Both HE and SCC compromise structural integrity but differ fundamentally in their mechanisms: HE is hydrogen-induced internal embrittlement, while SCC results from stress-assisted corrosion processes.
Fundamental Mechanisms of Hydrogen Embrittlement
Hydrogen embrittlement arises from the diffusion of atomic hydrogen into metals, leading to the formation of brittle hydrides or the initiation of microvoids and cracks at grain boundaries or inclusions. This phenomenon involves mechanisms such as hydrogen-enhanced decohesion, hydrogen-enhanced localized plasticity, and hydride formation, which reduce ductility and fracture toughness. In contrast to stress corrosion cracking, hydrogen embrittlement does not require a corrosive environment but depends primarily on hydrogen ingress and trapping within the metal lattice.
Fundamental Mechanisms of Stress Corrosion Cracking
Stress corrosion cracking (SCC) occurs due to the combined influence of mechanical stress and a corrosive environment, leading to localized anodic dissolution and embrittlement at the crack tip. This process involves the interaction of tensile stress with specific corrosive species, which facilitates crack initiation and propagation along grain boundaries or specific crystallographic planes. Unlike hydrogen embrittlement, which is driven primarily by hydrogen atom diffusion causing internal embrittlement, SCC fundamentally depends on the synergistic effects of corrosive electrochemical reactions and mechanical stresses.
Material Susceptibility and Environmental Factors
Hydrogen embrittlement primarily affects high-strength steels and nickel-based alloys due to their microstructural susceptibility to hydrogen diffusion and trapping, while stress corrosion cracking (SCC) commonly occurs in stainless steels and aluminum alloys exposed to specific corrosive environments such as chlorides or caustic solutions. Environmental factors influencing hydrogen embrittlement include hydrogen charging conditions like cathodic protection and acidic media, whereas SCC risk increases in the presence of tensile stress combined with corrosive agents and elevated temperatures. Understanding the interaction between material microstructure and environmental conditions is critical for predicting failure modes and implementing effective mitigation strategies in industrial applications.
Comparison of Failure Modes and Fracture Characteristics
Hydrogen embrittlement (HE) involves hydrogen atoms infiltrating a metal's crystal lattice, causing localized brittleness and intergranular or transgranular fracture with brittle features. Stress corrosion cracking (SCC) arises from the combined effect of tensile stress and a corrosive environment, resulting in crack initiation and propagation along grain boundaries or within grains, often with branching and brittle or quasi-cleavage fracture surfaces. HE typically shows sudden, catastrophic failure without significant plastic deformation, while SCC exhibits slow crack growth with stress-assisted corrosion mechanisms.
Differences in Affected Metals and Alloys
Hydrogen embrittlement primarily affects high-strength steels, titanium alloys, and nickel-based alloys by causing a loss of ductility and premature failure under tensile stress. Stress corrosion cracking predominantly occurs in a broader range of metals such as stainless steels, aluminum alloys, and copper alloys when exposed to specific corrosive environments combined with tensile stress. The key difference lies in hydrogen embrittlement's reliance on hydrogen atom diffusion into susceptible metals, whereas stress corrosion cracking requires a corrosive environment that initiates and propagates cracks at the metal surface.
Detection Methods and Testing Techniques
Hydrogen embrittlement detection methods primarily involve electrochemical permeation tests, thermal desorption spectroscopy, and slow strain rate testing (SSRT) to evaluate susceptibility and hydrogen content within metals. Stress corrosion cracking (SCC) testing techniques emphasize techniques such as constant load or constant strain tests, along with use of focused ion beam (FIB) microscopy and acoustic emission monitoring to identify and characterize crack initiation and propagation under corrosive environments. Advanced non-destructive evaluation (NDE) tools like ultrasonic testing and scanning electron microscopy (SEM) are critical in distinguishing between hydrogen embrittlement and SCC damage mechanisms.
Industrial Case Studies and Real-World Implications
Hydrogen embrittlement and stress corrosion cracking (SCC) are critical failure mechanisms extensively documented in industrial case studies involving high-strength steels and metal alloys used in aerospace, automotive, and petrochemical industries. Real-world implications highlight that hydrogen embrittlement primarily causes sudden brittle fractures under tensile stress, while SCC involves crack propagation accelerated by corrosive environments combined with tensile stress. Preventative strategies derived from case studies emphasize stringent material selection, controlled environmental conditions, and surface treatments to mitigate both hydrogen-induced damage and corrosion-related cracking in critical infrastructure.
Prevention and Mitigation Strategies
Hydrogen embrittlement prevention involves controlling hydrogen exposure through protective coatings, material selection with low hydrogen diffusivity, and strict control of manufacturing processes to minimize hydrogen ingress. Stress corrosion cracking mitigation relies on reducing tensile stress via proper design, applying corrosion-resistant alloys, and maintaining environmental control such as pH adjustment and inhibitors to limit aggressive ion interaction. Both phenomena benefit from regular inspections and monitoring techniques like acoustic emission and electrochemical methods for early damage detection.
Future Trends in Research and Materials Engineering
Recent research in hydrogen embrittlement (HE) and stress corrosion cracking (SCC) emphasizes the development of advanced high-entropy alloys and nanostructured materials with enhanced resistance to atomic hydrogen diffusion and corrosive environments. Emerging techniques like in-situ electron microscopy and atom probe tomography allow for precise characterization of microstructural changes that precede failure, facilitating predictive modeling and material design. Integration of machine learning algorithms with experimental data accelerates the discovery of corrosion-resistant alloys tailored for aerospace, automotive, and energy sectors exposed to hydrogen-rich and chloride-laden conditions.
Intergranular fracture
Hydrogen embrittlement primarily causes intergranular fracture by trapping hydrogen at grain boundaries, whereas stress corrosion cracking involves intergranular fracture due to synergistic effects of tensile stress and corrosive environments weakening grain boundary cohesion.
Atomic hydrogen diffusion
Atomic hydrogen diffusion accelerates hydrogen embrittlement by penetrating metal lattices and causing internal fractures, whereas stress corrosion cracking involves the combined effect of tensile stress and corrosive environments leading to crack propagation without necessarily relying on atomic hydrogen diffusion.
Anodic dissolution
Anodic dissolution plays a critical role in stress corrosion cracking by accelerating metal ion loss at crack tips, whereas hydrogen embrittlement primarily involves hydrogen-induced lattice weakening without significant anodic metal dissolution.
Cathodic protection
Cathodic protection mitigates hydrogen embrittlement by reducing hydrogen ion reduction on metal surfaces, whereas it can exacerbate stress corrosion cracking by promoting anodic dissolution in susceptible alloys.
High-strength alloys
High-strength alloys are highly susceptible to hydrogen embrittlement, which involves hydrogen-induced loss of ductility, while stress corrosion cracking occurs due to the combined effects of tensile stress and corrosive environments leading to crack propagation.
Delayed failure
Hydrogen embrittlement causes delayed failure by hydrogen atoms diffusing into metals and weakening atomic bonds, whereas stress corrosion cracking results from the combined effects of tensile stress and corrosive environments leading to crack propagation.
Protective coatings
Protective coatings mitigate hydrogen embrittlement by preventing hydrogen ingress and reduce stress corrosion cracking by isolating the metal surface from corrosive environments.
Residual tensile stress
Residual tensile stress significantly accelerates hydrogen embrittlement by promoting hydrogen diffusion and accumulation in metal lattice, whereas in stress corrosion cracking, it enhances crack initiation and propagation by facilitating anodic dissolution under corrosive conditions.
Crack propagation kinetics
Hydrogen embrittlement accelerates crack propagation kinetics by facilitating hydrogen diffusion and inducing brittle fracture mechanisms, whereas stress corrosion cracking propagates cracks through synergistic effects of tensile stress and corrosive environments causing intergranular or transgranular corrosion-assisted crack growth.
Sulfide stress cracking
Sulfide stress cracking, a form of hydrogen embrittlement, primarily occurs in high-strength steels exposed to hydrogen sulfide environments, causing brittle fracture due to hydrogen diffusion and sulfide-induced corrosion.
Hydrogen embrittlement vs stress corrosion cracking Infographic
 njnir.com
njnir.com